In June 2023, BioNTech presented the first human trial data of BNT211 at the American Society of Clinical Oncology (ASCO). BNT211 is a CLDN-6-targeting CAR-T cell therapy in combination with a CLDN6-encoding mRNA vaccine (CARVac). mRNA vaccines stimulate the expansion of CAR-T cells in the patients’ body by inducing the expression of CLDN6 on the surface of antigen-presenting cells, thereby enhancing the efficacy of CAR-T cell therapy. The latest study data of BNT211 showed that CLDN6 CAR-T infusion alone or in combination with CARVac was safe and displayed promising efficacy in patients with CLDN6-positive cancers, and the results were even more encouraging when compared to data obtained from other trials targeting solid tumors with CAR T-cell therapies.
Will CLDN6 be the next promising target for solid tumor treatment after ClDN18.2? Which pharmaceutical companies work in the pipeline targeting CLDN6?
What is the structure and function of CLDN6?
What is the role of CLDN6 in solid tumors?
Which pharmaceutical companies work in the pipeline targeting CLDN6?
DIMA’s CLDN6 functional active protein
1. What is CLDN6?
Claudin 6, also known as CLDN6, is a member of the claudins (CLDNs) family. In 1998, Furuse Mikio cloned proteins with a relative molecular mass of about 22 kDa from chicken liver and named them claudin-1 (211 AA) and claudin-2 (230 AA) respectively [1]. These findings marked the discovery of the first members of the claudin family. Since then, a total of 27 Claudin family members have been discovered, with 24 of them expressed in mammals. Claudins are mainly expressed in endothelial or epithelial cells in a tissue-specific manner. According to the degree of sequence homology, claudins can be classified into two types, classical and non-classical claudins. Claudins are a representative transmembrane protein family in tight junctions (TJ), which play crucial roles in maintaining the unique fence and barrier properties of TJ.
CLDN6 belongs to the classical claudins and is homologous to CLDN1, CLDN2, CLDN3, CLDN4, CLDN5, CLDN7, CLDN8, CLDN9, CLDN10, CLDN14, CLDN15, CLDN17 and CLDN19 [2]. 2001, Turksen K. et al. identified CLDN6 from a differential display analysis of differentiating embryoid bodies (EBs), although its cDNA was first isolated from an ectoderm-specific library by Harrison S.M.’s team in 1995 [3] [4].
The expression of CLDN6 is dynamically regulated by many factors. CLDN6 is generally expressed in fetal tissues such as stomach, pancreas, lung, and kidney, but not in corresponding adult tissue samples [5]. CLDN6 is one of the first epithelial fate-determining protein expressed in embryonic stem (ES) cells and a cell surface-specific marker of human pluripotent stem cells (hPSCs) [6].
2. What is the structure and function of CLDN6?
The human CLDN6 protein consists of 220 amino acids with a molecular weight of 23kDa, the gene of which is located on chromosome 16p13.3. As shown in Figure 1, like other CLDNs, CLDN6 has four transmembrane domains, a short cytoplasmic N-terminus, a C-terminal cytoplasmic domain, two extracellular domains (larger ECL1 and a smaller ECL2) and a short intracellular loop. ECL1 and the PDZ-binding domain at the cytoplasmic C-terminus are characteristic domains of CLDNs. ECL1 crosses between cells to form ion-selective pores. The amino acid positions and charges on the ECL1 region vary in different claudins, and the C-terminal sequence is another point to distinguish the members of claudins. Claudins can directly act on PDZ-containing ZO-1, ZO-2, ZO-3, and MUPP1 in the cytoplasm by its C-terminal PDZ binding sequence and some potential phosphorylation sites. ZO-1 and ZO-2 can directly connect with actin filaments, enabling TJ protein and intracellular skeleton structures to form a stable system.
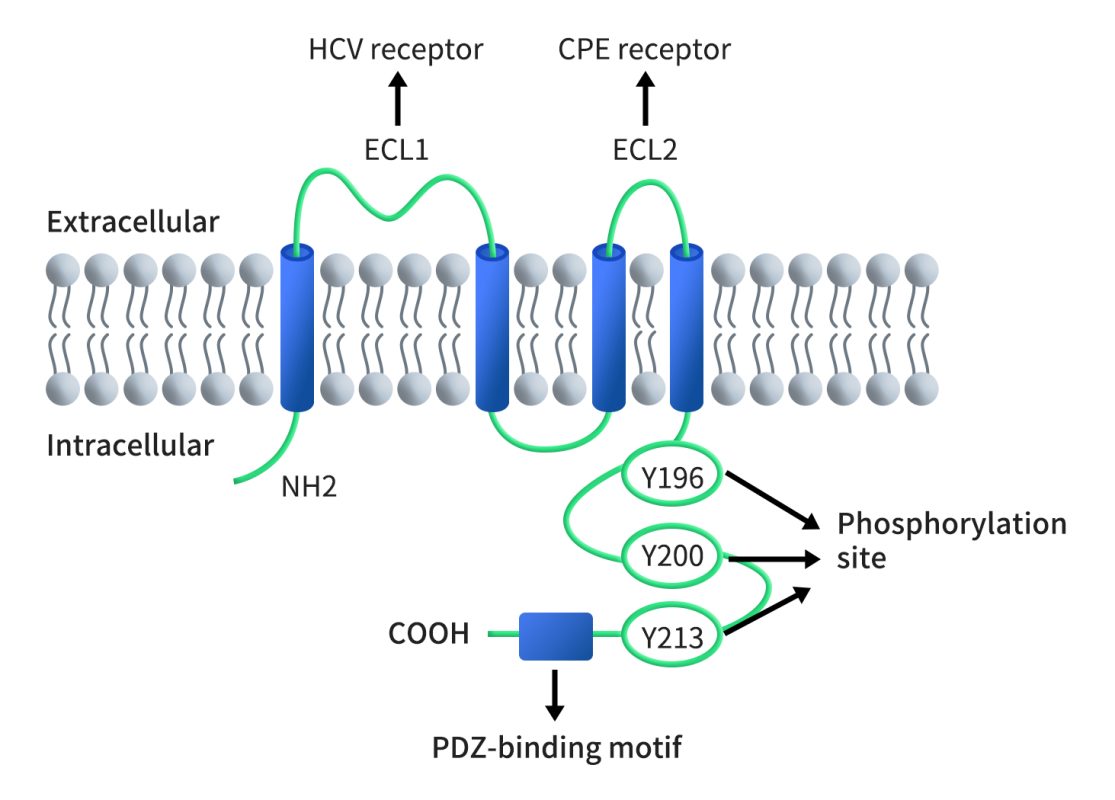
Figure 1. The structure of CLDN6 [5]
As a member of the claudin protein family, CLDN6 also has the functions of tight junction permeability regulation and barrier formation. In terms of permeability regulation, CLDN6 is critical for controlling the permeability of chloride ion in neonatal renal proximal tubules and regulation of ion transport in the lymphatic sac epithelium of the inner ear [7][8]. CLDN6 is also important for maintaining the lung epithelial barrier. It helps to prevent the entry of harmful substances and pathogens into the lungs. In addition, CLDN6 is significantly involved in the formation of the epidermal permeability barrier (EPB) during preterm birth. Unlike other claudin members, CLDN6 is the only CLDN with potential specificity because it activates cell adhesion signals and regulates the activity of nuclear receptors. The expression and activity of CLDN6 can be regulated by stimuli and transcription factors, DNA methylation, phosphorylation and palmitoylation. These regulatory mechanisms highlight the dynamic nature of CLDN6 and emphasize its versatility in responding to various cellular and physiological cues.
3. What is the role of CLDN6 in solid tumors?
Tumor cells often exhibit loss of intercellular tight junctions, which is important in the development of tumor metastasis. Altered tight junction proteins lead to increased permeability, allowing more nutrients and other factors to infiltrate the tumor and promote its growth. As the main structure of tight junctions, abnormal expression of Claudins can lead to structural damage and functional impairment of epithelial cells and endothelial cells, which has been observed in various epithelial-origin tumors. For example, Claudin2 is down-regulated in prostate cancer and breast cancer, Claudin3 in kidney cancer and bladder cancer, and Claudin4 in kidney cancer and liver cancer. Unlike other CLDNs, CLDN6 is a tumor-specific protein that is present in a variety of solid tumors, including ovary, endometrium, lung, stomach, and testis, but not in healthy adult tissues. As a result, there has been an increasing focus on studying the role of CLDN6 in cancer, and it has emerged as a promising target for tumor treatment.
The mechanism of CLDN6 in tumors is not yet fully understood, and there is no universally recognized pathway. Take breast cancer as an example, a study by the research team from Jilin University confirmed that the downregulation of CLDN6 can be mediated through DNA methyltransferase 1 (DNMT1 ) and its DNA methylation, which is dependent on the SMAD2 pathway. Inhibition of CLDN6 methylation by down-regulating DNMT1 expression suppresses epithelial-to-mesenchymal transition (EMT), migration, and invasion of breast cancer cells [9]. Before this study, they also proved that claudin-6 gene silencing can enhance the proliferation and migration of breast cancer cells, and at the same time increase the activity of MMP-2 through the p38 MAPK signaling pathway, further promoting the malignant progression of human breast cancer [10]. In addition, several studies have suggested that the expression of CLDN6 in breast cancer cells is regulated by multiple signaling pathways, including the HIF-1α pathway [11], the ASK1-p38/c-Jun N pathway [12] and (Erβ)/Erα- Beclin1 pathway [13]. These findings highlight the significance of CLDN6 in tumor development and provide valuable insights into potential therapeutic strategies targeting CLDN6 in cancer treatment.
4. Which pharmaceutical companies work in the pipeline targeting CLDN6?
Currently, there is a wide range of targeted CLDN6 immunotherapies being developed for the treatment of cancer, including monoclonal antibodies, bispecific antibodies (BsAbs) and antibody-drug conjugates (ADCs), etc. As of today, there are 7 clinical projects underway, including 1 monoclonal antibody, 2 double-antibodies, 2 ADC drugs, and 2 CAR-T therapies. These clinical projects demonstrate the increasing interest and potential in CLDN6-targeted immunotherapies for the treatment of cancer. The diverse range of approaches being explored reflects the complexity of cancer and the need for multifaceted strategies to effectively combat the disease.
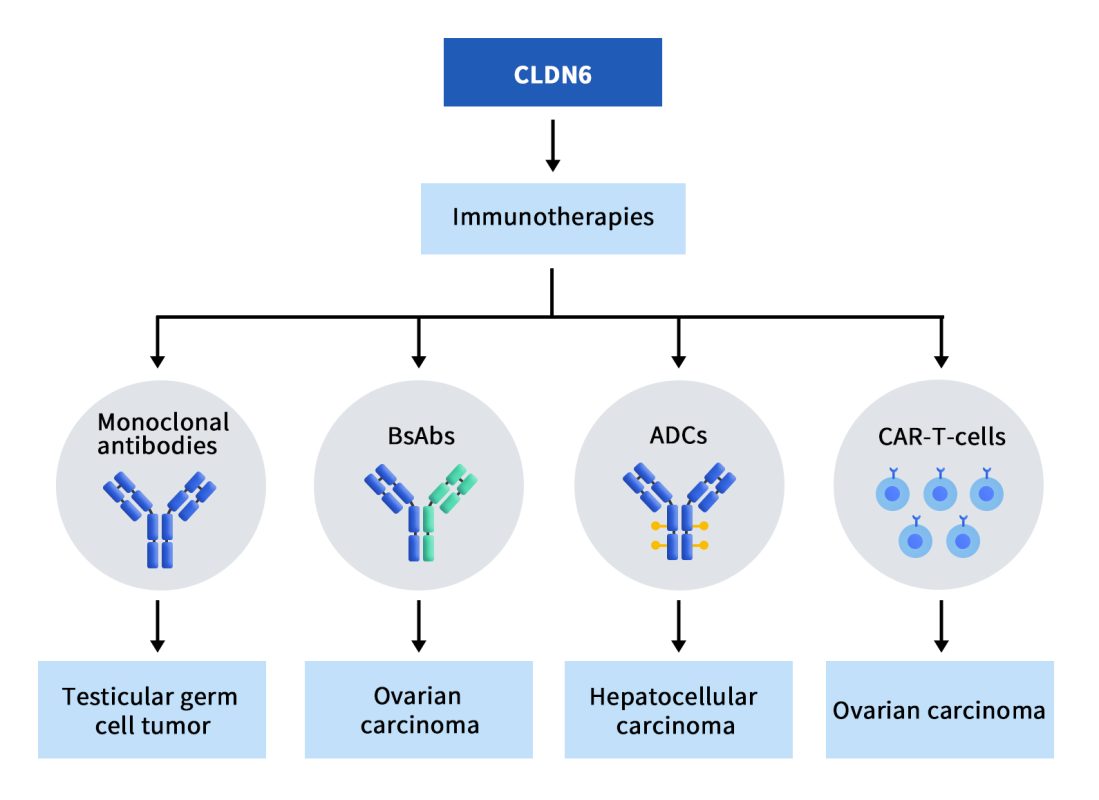
Figure 2. The types of drugs targeting CLDN6 [5]
4.1 ASP1650
ASP1650 is a chimeric mouse/human IgG1 antibody targeting CLDN6 developed by Astellas. It has been categorized as the fastest-growing CLDN6 monoclonal antibody, but it is also terminated early due to unsatisfactory results from Phase II clinical trials. The clinical results published in 2022 showed that ASP1650 did not demonstrate clinically meaningful single-agent activity in relapsed/refractory testicular germ cell tumor (GCT) patients (19 males), and the pipeline has been discontinued [14].
4.2 BNT211&BNT142
BioNTech, a German pharmaceutical company, announced several updates on its CLDN product portfolio at the 2023 ASCO Annual Meeting, including BNT211 and BNT142. Among them, the most striking is the latest research results of BNT211 for the treatment of solid tumors.BNT211 consists of two parts, one is an autologous CAR-T cell therapy targeting CLDN-6, and the other is a CLDN6-encoded mRNA vaccine (CARVac) developed based on BioNTech’s proprietary mRNA-lipoplex technology. The therapy is designed to induce robust immune responses against various CLDN6-positive solid tumors, such as ovarian, sarcoma, testicular, endometrial, and gastric cancers.
BNT211 is currently being evaluated in a Phase 1/2 trial, either as a standalone treatment or in conjunction with CARVac, for patients with CLDN6-positive relapsed/refractory advanced solid tumors. Encouragingly, the latest results from this trial have shown positive activity and manageable safety outcomes. Out of the 17 patients who received BNT211 with or without CARVac, the overall objective response rate (ORR) was 41%, and the disease control rate (DCR) reached 65%. Notably, a patient with testicular cancer who received automated BNT211 treatment achieved surgical complete response (sCR). Five patients achieved partial response (PR) and three patients achieved stable disease (SD). BNT211 maintained its efficacy during the automated manufacturing process, which supports the scale-up of the manufacturing process [15].
BNT142 is a mRNA vaccine encoding a CD3 x CLDN6 bispecific antibody. After intravenous injection, BNT142 is directed to the liver, where the two RNAs are translated, assembled, and secreted into the circulation as antibodies. Reports of hepatotoxicity are important because mRNA vaccine toxicity tends to accumulate in the liver. BNT142 is currently undergoing Phase I dose-escalation clinical trials to evaluate its safety and determine the optimal dosage.
4.3 TORL-1-23
TORL-1-23 is an antibody-drug conjugate (ADC) developed by TORL BioTherapeutics which conjugates anti-CLDN6 monoclonal antibody and tubulin inhibitor MMAE through a cleavable linker. In vitro experiments show that TORL-1-23 is highly selective, and it can selectively and strongly bind to CLDN6 overexpressing cell lines, but not to other claudin overexpressing cell lines, such as CLDN3, CLDN4, and CLDN9. The TORL-1-23 clinical phase I trial results announced at the ASCO annual meeting showed that among 25 refractory cancer patients who received an average of 5 previous treatments, 28% of the patients achieved confirmed remission. The company plans to enroll more patients with CLDN6-positive ovarian cancer and non-small cell lung cancer and expand to other CLDN6-positive cancer types after determining the recommended dose for Phase 2 clinical trials.
4.4 DS-9606
DS-9606 is a CLDN6-targeting ADC constructed by Daiichi Sankyo based on benzodiazepine derivatives (PBD). The results of preclinical research data show that the DS-9606 exhibits significant anti-tumor activity. DS-9606 is currently undergoing a phase 1 clinical study, which aims to evaluate the safety and tolerability of DS-9606a in patients with advanced solid tumors. Apart from safety, the study also intends to evaluate the pharmacokinetic properties of DS-9606a, investigate the duration of response and progression-free survival of DS-9606a, and assess the immunogenicity of DS-9606a.
4.5 CLDN6-CAR-NK cell therapy
CLDN6-CAR-NK cell therapy is a phase 1/2 clinical trial currently being carried out at the Second Affiliated Hospital of Guangzhou Medical University, and no results have been announced yet. The study focuses on advanced cancer patients with Claudin6 expression. PBMCs were collected from the patients and NK cells were isolated. Following this, NK cells were genetically modified to express the Claudin6-targeted CAR, and the number of modified NK cells was expanded as needed for the trial. The quality and killing activity of the cells were then assessed before being transplanted back into the patient by systemic or local infusion. Close monitoring is conducted throughout the process to gather relevant data and outcomes.
4.6 AMG 794
AMG 794 is a bispecific antibody with a prolonging half-life developed based on the bispecific T cell engager (BITE®) technology platform, which can bind to human and cynomolgus monkey CLDN6 and CD3. AMG 794 can redirect T cells to kill tumor cells expressing CLDN6. It is currently in the stage of clinical phase I recruitment of patients.
5. DIMA’s CLDN6 functional active protein
CLDN6 is closely related to Claudins (including CLDN3, CLDN4, and CLDN9), and there is a genetic overlap between them, which poses a challenge to the development of CLDN6-targeted therapies. Unlike CLDN6, these closely related proteins are expressed in normal healthy tissue, so it is critical that the drug be selective for CLDN6. Any kind of immunotherapy is inseparable from high-quality antigens. DIMA Biotech provides various CLDN6 proteins to meet customers’ needs, here we display validated data of the most popular one as follows:
- Human CLDN6 full-length protein-synthetic nanodisc (Cat. FLP100008)
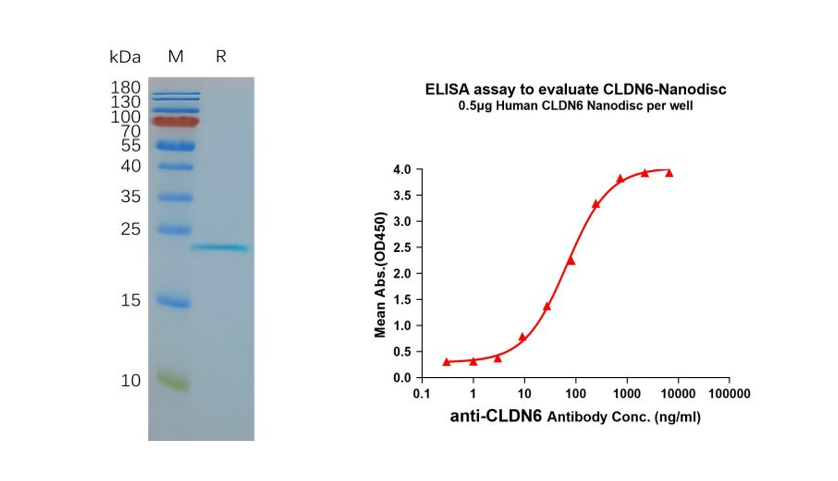
Figure 3. Validation data of purified full-length human CLDN6 Nanodisc (Cat. FLP100008). Human CLDN6-Nanodisc, Flag Tag on SDS-PAGE (left); CLDN6-Nanodisc (Cat. FLP100008) can bind to anti-CLDN6 mAb (Cat. BME100082) and the EC50 is 66.99ng/ml (right).
- All the CLDN6-related products supplied by DIMA Biotech
| Product types | Cat# | Product name |
| ECD protein | PME100063 | Human CLDN6 Protein, mFc Tag |
| ECD protein | PME101206 | Human CLDN6(29-81) Protein, hFc Tag |
| ECD protein | PME101188 | Human CLDN6(138-160) Protein, hFc Tag |
| ECD protein | PME101189 | Human CLDN6(138-160) Protein, mFc Tag |
| ECD protein | PME101207 | Human CLDN6(138-160) Protein, hFc Tag |
| Full-length membrane protein | FLP100038 | Human CLDN6 full length protein-VLP |
| Full-length membrane protein | FLP100004 | Human CLDN6 full length protein -MNP |
| Full-length membrane protein | FLP100008 | Human CLDN6 full length protein-synthetic nanodisc |
| Reference Antibody | BME100082 | Anti-CLDN6 (IMAB027) mAb |
References:
[1] Furuse M, Fujita K. Claudin 1 and -2; novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin [J].Cell Biol,1998,141(7);1539-1550.[2] Günzel D., Fromm M. Claudins and other tight junction proteins. Compr. Physiol. 2012;2:1819–1852.
[3] Turksen K., Troy T.C. Claudin-6: A novel tight junction molecule is developmentally regulated in mouse embryonic epithelium. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2001;222:292–300.
[4] Harrison S.M., Dunwoodie S.L., Arkell R.M., Lehrach H., Beddington R.S. Isolation of novel tissue-specific genes from cDNA libraries representing the individual tissue constituents of the gastrulating mouse embryo. Development. 1995;121:2479–2489.
[5] Qu H, Jin Q, Quan C. CLDN6: From Traditional Barrier Function to Emerging Roles in Cancers. Int J Mol Sci. 2021 Dec 14;22(24):13416.
[6] Ben-David U, Nudel N, Benvenisty N. Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nat Commun. 2013;4:1992.
[7] Sas D, Hu M, Moe OW, Baum M. Effect of claudins 6 and 9 on paracellular permeability in MDCK II cells. Am J Physiol Regul Integr Comp Physiol. 2008 Nov;295(5):R1713-9.
[8] Kitajiri SI, Furuse M, Morita K, Saishin-Kiuchi Y, Kido H, Ito J, Tsukita S. Expression patterns of claudins, tight junction adhesion molecules, in the inner ear. Hear Res. 2004 Jan;187(1-2):25-34.
[9] Lu Y, Wang L, Li H, Li Y, Ruan Y, Lin D, Yang M, Jin X, Guo Y, Zhang X, Quan C. SMAD2 Inactivation Inhibits CLDN6 Methylation to Suppress Migration and Invasion of Breast Cancer Cells. Int J Mol Sci. 2017 Aug 30;18(9):1863.
[10] Ren Y, Wu Q, Liu Y, Xu X, Quan C. Gene silencing of claudin-6 enhances cell proliferation and migration accompanied with increased MMP-2 activity via p38 MAPK signaling pathway in human breast epithelium cell line HBL-100. Mol Med Rep. 2013 Nov;8(5):1505-10.
[11] Jia Y, Guo Y, Jin Q, Qu H, Qi D, Song P, Zhang X, Wang X, Xu W, Dong Y, et al. A SUMOylation-dependent HIF-1α/CLDN6 negative feedback mitigates hypoxia-induced breast cancer metastasis. J Exp Clin Cancer Res. 2020;39:42.
[12] Guo Y, Lin D, Zhang M, Zhang X, Li Y, Yang R, Lu Y, Jin X, Yang M, Wang M, et al. CLDN6-induced apoptosis via regulating ASK1-p38/JNK signaling in breast cancer MCF-7 cells. Int J Oncol. 2016;48:2435–2444.
[13] Song P, Li Y, Dong Y, Liang Y, Qu H, Qi D, Lu Y, Jin X, Guo Y, Jia Y, et al. Estrogen receptor β inhibits breast cancer cells migration and invasion through CLDN6-mediated autophagy. J Exp Clin Cancer Res. 2019;38:354.
[14] Adra N, Vaughn DJ, Einhorn LH, Hanna NH, Funt SA, Rosales M, Arozullah A, Feldman DR. A phase II study assessing the safety and efficacy of ASP1650 in male patients with relapsed refractory germ cell tumors. Invest New Drugs. 2022 Oct;40(5):1087-1094.
[15] Mackensen A, Haanen JBAG, Koenecke C, et al. CLDN6 CAR-T cell therapy of relapsed/refractory solid tumors ± a CLDN6-encoding mRNA vaccine: dose escalation data from the BNT211-01 phase 1 trial using an automated product. J Clin Oncol. 2023;41(suppl 16):2518.
