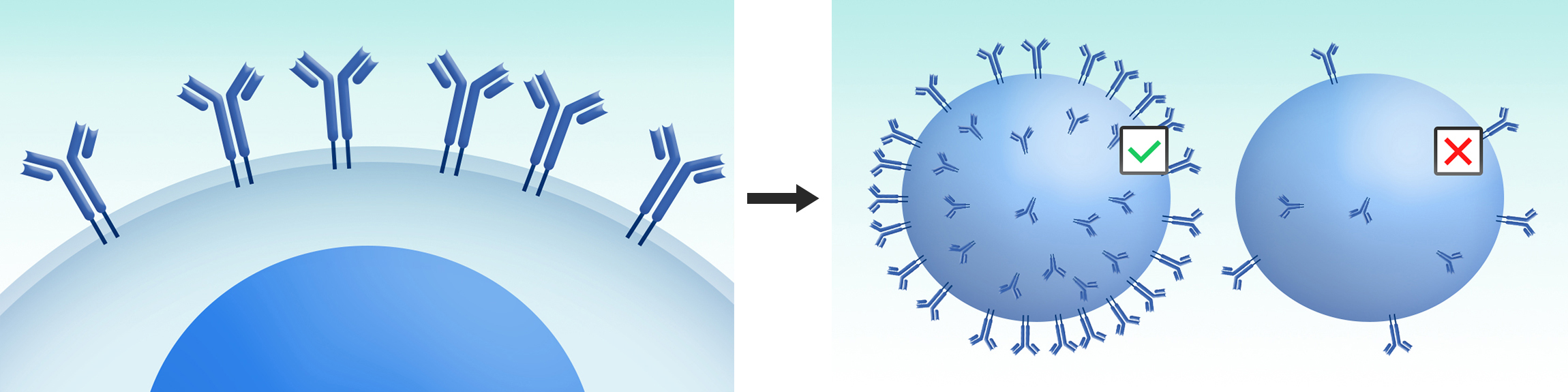
Cells exhibited high antibody surface expression level will be selected.
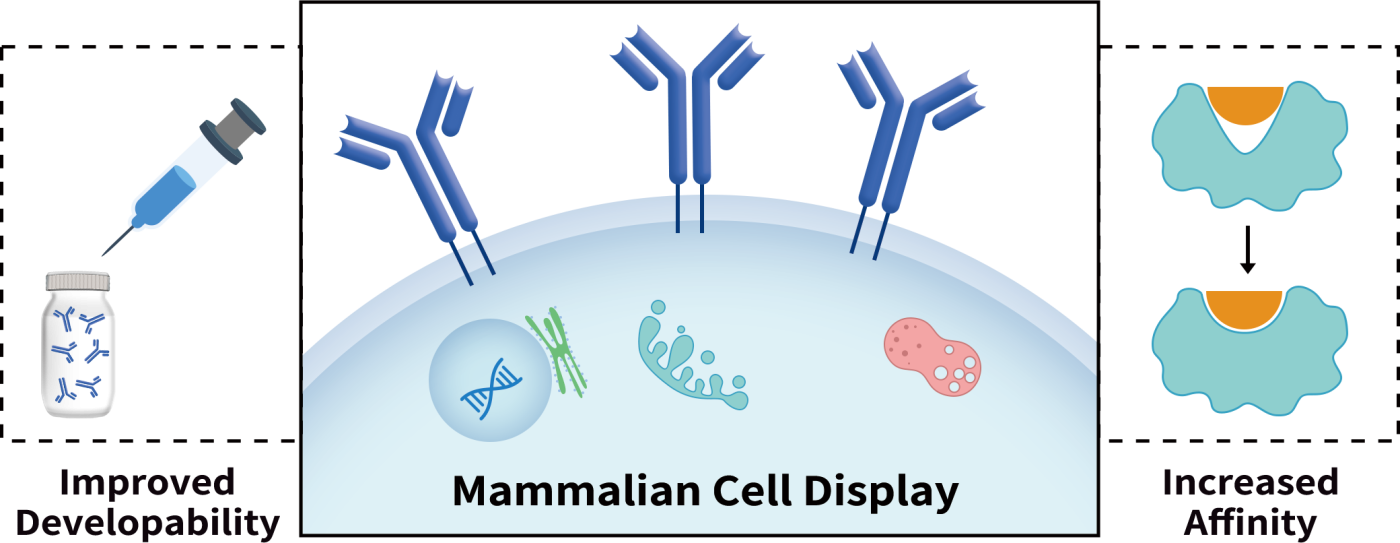
Antibody Affinity Maturation — Through Mammalian Cell Display Technology
Antibody drug exerts its therapeutic property by binding to its target. Most of the time, screening for antibodies with stronger affinity is a prerequisite for lead drug candidate optimization. However, to develop a good lead antibody drug for clinical trial, many other aspects on the candidate molecule also need to be considered. These include target specificity and developability, etc.
Currently, phage and yeast display platforms are the most commonly used technologies for antibody affinity improvement. Unfortunately, they all have intrinsic issues due to the expression system used to display antibodies. Mammalian display is a challenging, but most suitable platform for therapeutic antibody drug development.
DiLibraryTM developed by DIMA Biotech is designed to screen ideal molecules from a large number of antibody variants expressed on mammalian cell surface. This system is delicately designed to not only screen out molecules with the strongest binding property, but also the molecules with best biological compatibilities with downstream development. With this system, the full length antibody variant library designed and constructed by AI algorithm is displayed on mammalian cell surface. Through FACS sorting, the cells expressing high affinity binders will be fished out and cloned.
All the antibodies displayed on mammalian cell surface will go through cellular secretion pathway and carry most authentic PTMs. During this cellular display procedure, those molecules with certain issues on developability, such as aggregation tendency, structural instability, high hydrophobicity, low expressors, etc, will be ruled out by the mammalian cellular quality control system or screening. Therefore, DiLibraryTM is a superior system for therapeutic antibody drug engineering.
Based on mammalian cell display

Optimized by AI design
Great developability

Fast delivery
Developability Assurance
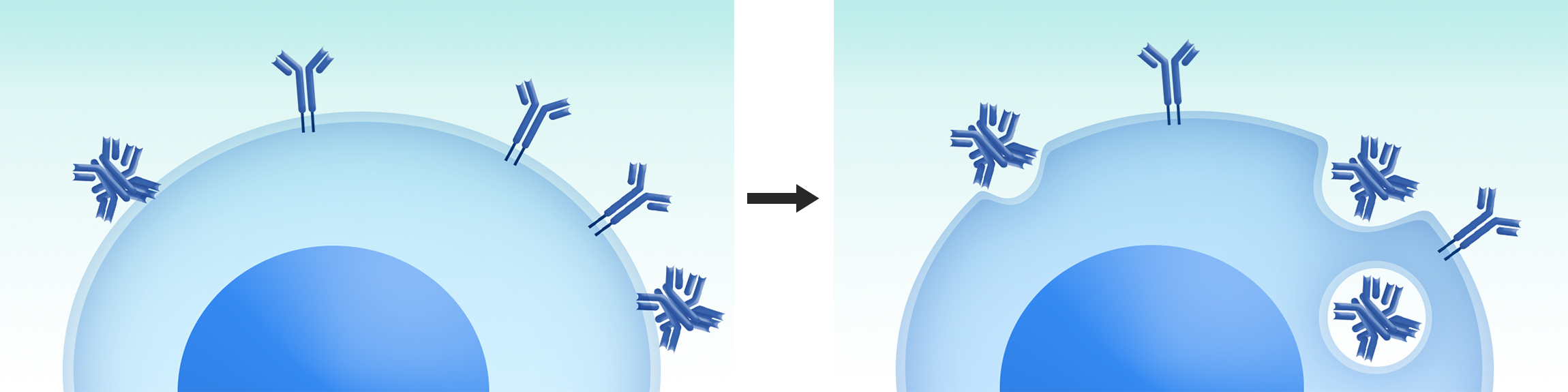
Antibody molecules with structural instabilities, such as high aggregation tendency, improper folding, etc,
will be cleared by cellular quality control system and ruled out.
Service Details
| Procedures | SERVICE DETAILS | ESTIMATED PRODUCTION CYCLE (WEEK) |
|---|---|---|
| Library construction | Antibody expression report. Library binding report. | 1-2 weeks |
| Cell sorting and affinity ranking | Cell sorting report. Affinity ranking report. | 2-3 weeks |
| Cloning and sequencing | Sequences of high affinity antibodies. | 2-3 weeks |
| Antibody production and affinity measurement | QC report of selected antibodies. SPR report of selected antibodies. | 2 weeks |
| PTM optimization | Analysis of risky PTM sites. Antibody sequences with risky PTM sites optimized. | Optional |
Case study with DiLibraryTM platform
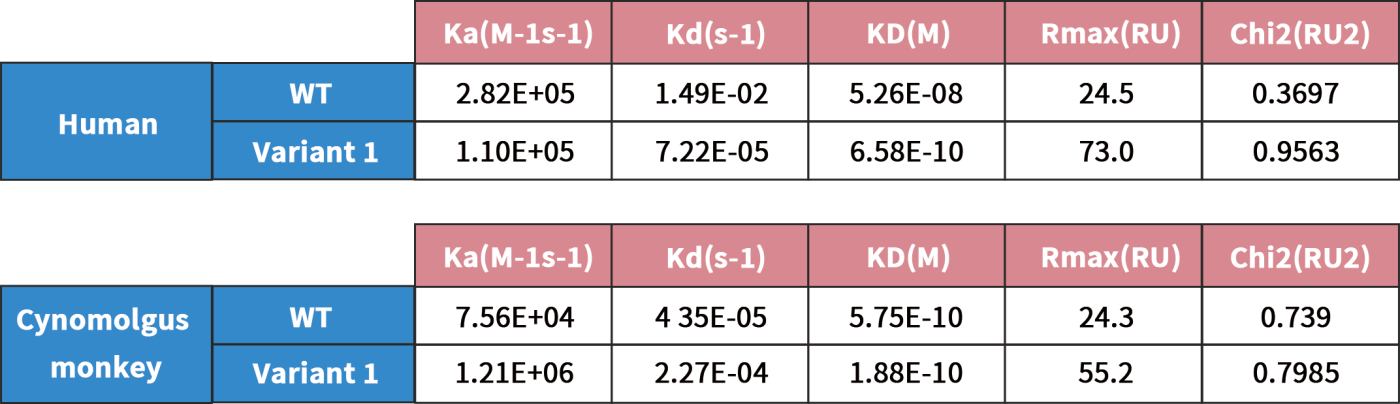
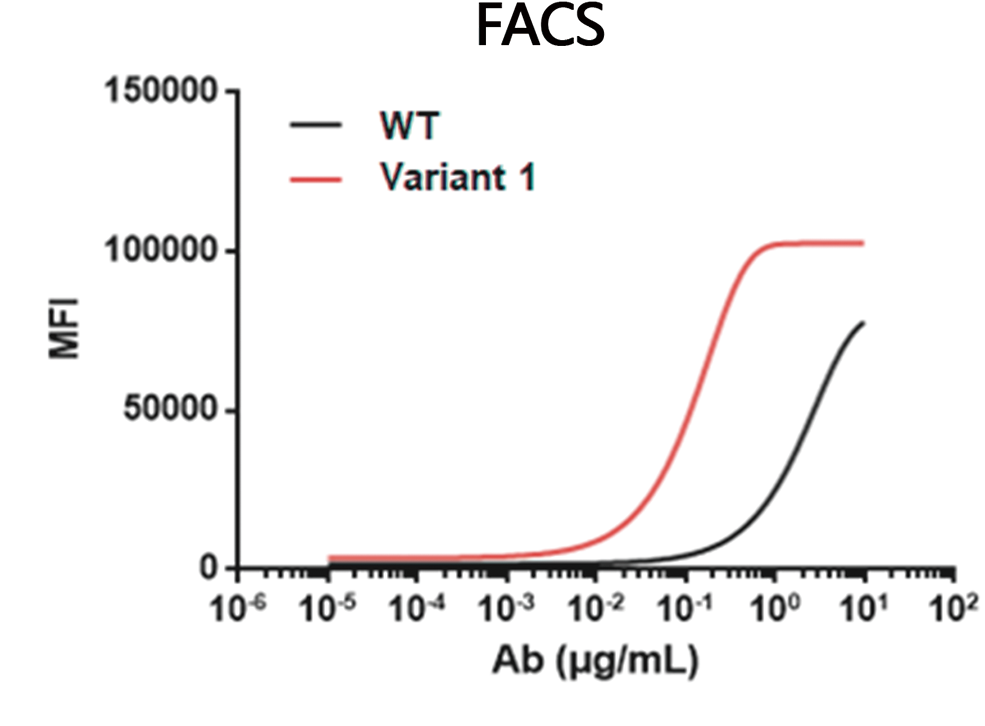
In this study, we chose a humanized anti-CCR8 antibody as a testing example. The original clone is suboptimal in binding affinity. To improve its binding affinity towards both human and cyno CCR8 protein, affinity maturation was carried out by using DIMA’s DiLibrary technology. Through SPR affinity measurement and other binding assays (ELISA and Flow cytometry), one of the isolated variant clones exhibits around 100 times improvement on binding affinity than original clone. In the meantime, the selected clone gains several fold increases on binding affinity towards cyno CCR8. Sequence analyses demonstrates that two single amino acid change are identified on LCDR3 and HCDR3 of parental molecule.
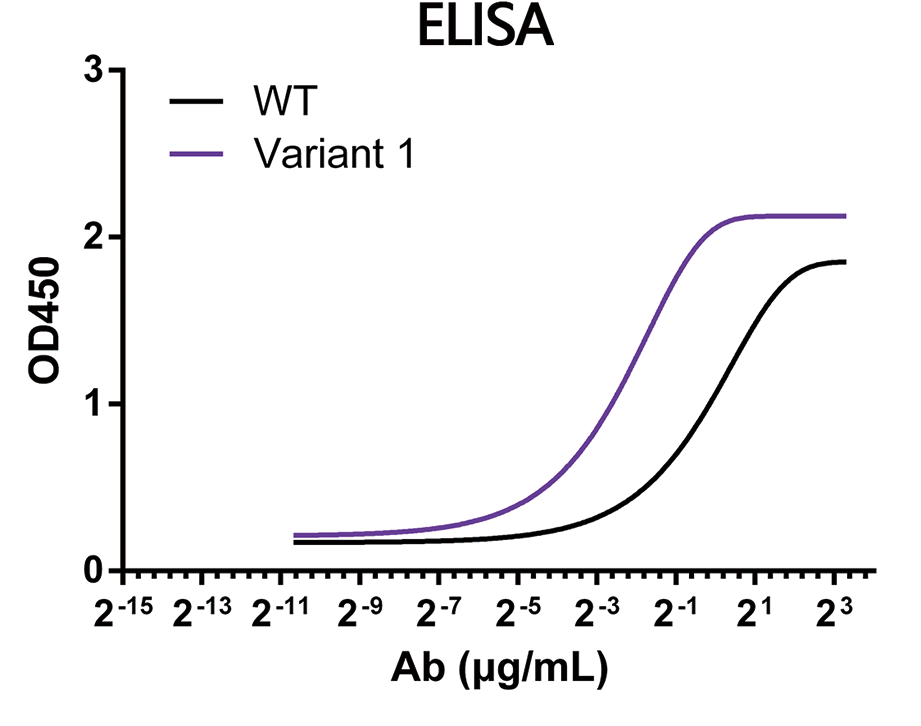
SPR data shows that the selected clone exhibits 100 times higher binding affinity to human CCR8 protein than its parental molecule.