Human carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5), initially discovered in colon cancer by Gold and Freeman in 1965 [1] [2], has long served as a pivotal tumor marker for clinical detection and diagnosis of colorectal cancer. Recent advancements in CEACAM5 research have unveiled its potential as a target for developing novel cancer therapeutics. Notably, at the 2023 AACR meeting, Sanofi and LaNova Pharmaceuticals announced significant progress in their respective antibody-drug conjugate (ADC) and dual antibody research targeting CEACAM5. So what is CEACAM5? What are the characteristics of its expression? What are the current pipelines targeted CEACAM5? What indications are involved?
1. What is the structure and distribution of CEACAM5?
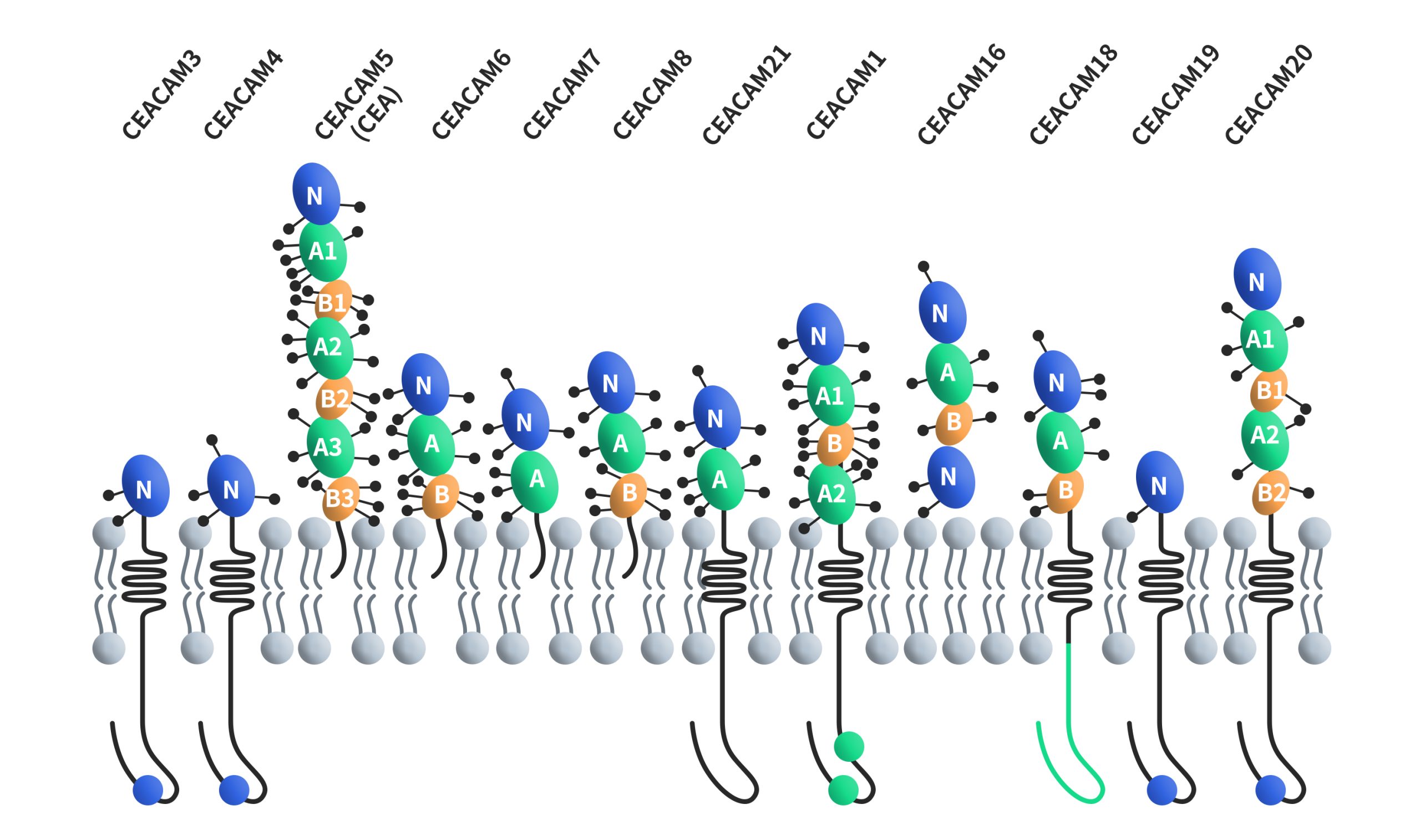
CEACAMs, short for carcinoembryonic antigen-related cell adhesion molecules, constitute a family of cell surface glycoproteins encompassing 12 distinct members: CEACAM1, CEACAM3, CEACAM4, CEACAM5, CEACAM6, CEACAM7, CEACAM8, CEACAM16, CEACAM18, CEACAM19, CEACAM20 and CEACAM21. These proteins are classified within the immunoglobulin superfamily of adhesion molecules, and they share structural similarities with unique variations. Except for CEACAM16, all CEACAMs feature a 108-amino-acid immunoglobulin variable region (IgV)-like domain at their N-terminus. CEACAM16 stands out by possessing two IgV-like domains at its N-terminus. Furthermore, the number of immunoglobulin constant region (IgC) type 2-like domains at the N-terminus varies across CEACAM members, ranging from 0 to 6. All CEACAMs are tethered to the cell membrane, with distinct mechanisms of membrane attachment. CEACAM5, CEACAM6, CEACAM7 and CEACAM8 are bound to the cell membrane through GPI, while CEACAM1, CEACAM3, CEACAM4, CEACAM19, CEACAM20 and CEACAM21 are anchored to the cell membrane through transmembrane domains. CEACAM16 is a secreted form without any membrane anchorage.
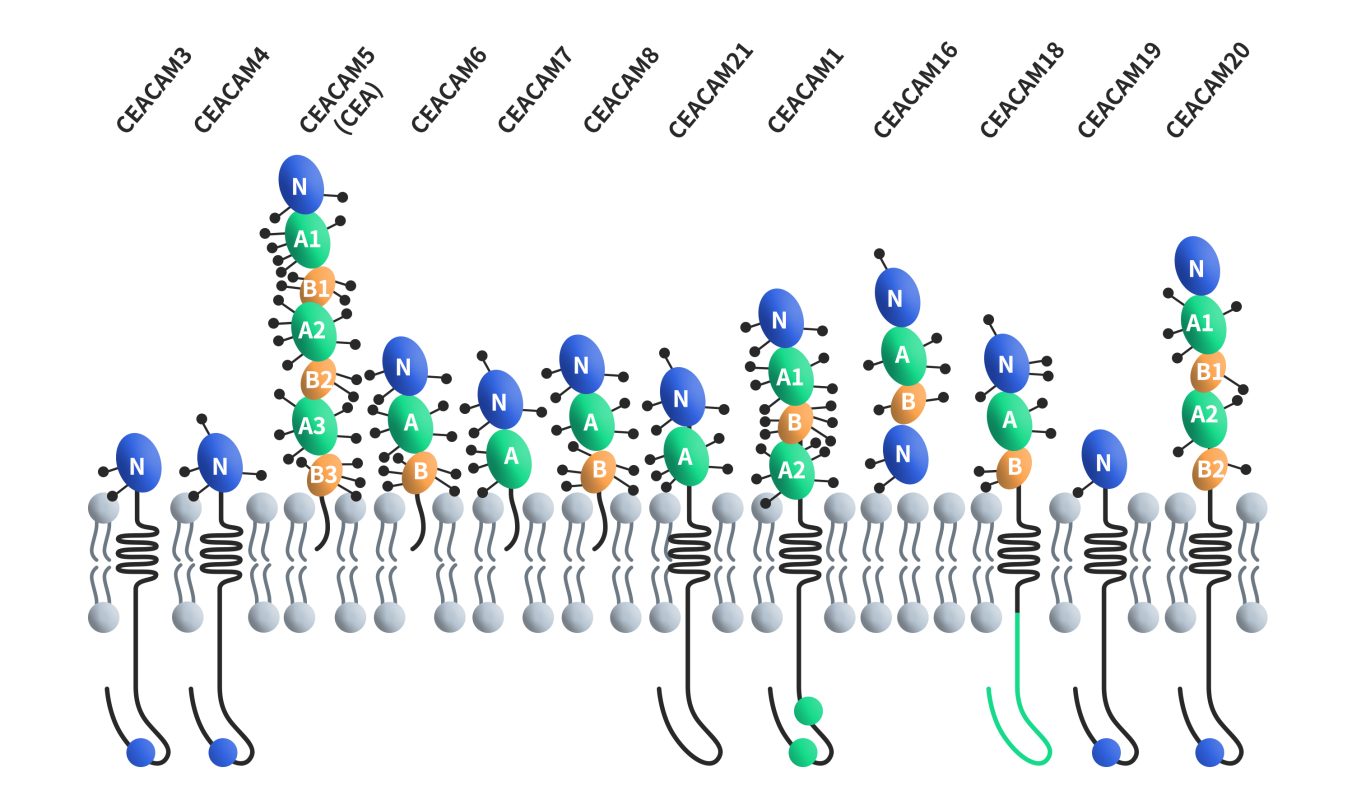
Figure 1. The structures of CEACAMs [4]
CEACAM5, also known as CEA (carcinoembryonic protein) or CD66e, is the first discovered member of the carcinoembryonic antigen-related cell adhesion molecule (CEACAMs) family. The human CEACAM5 gene is precisely located on chromosome 19q13.2 and spans approximately 21 kilobases, encompassing nine exons and three non-coding exons. The CEACAM5 protein comprises 642 amino acids, with an estimated molecular weight of around 70 kDa, and it boasts an impressive 28 potential N-linked glycosylation sites. This glycoprotein carries 24-26 asparagine-linked sugar chains, ultimately culminating in a final molecular weight of approximately 180 kDa. The protein binds to the membrane through glycosylphosphatidylinositol (GPI) and includes seven extracellular immunoglobulin-like domains, which involve one variable (lgV)-like domain and six lgC-like domains (A1-B3), there is a 34-amino acid signal peptide in front of the lg domain [3] [4].
In contrast to other CEACAMs expressed in hematopoietic cells like CEACAM1, CEACAM8, and CEACAM6, CEACAM5 remains notably absent in hematopoietic cells. Research findings highlight that CEACAM5 expression commences during the early stages of human embryonic and fetal development, particularly between 9 to 14 weeks, and persists throughout an individual’s lifetime in specific cell types. In adult normal tissues, CEACAM5 is predominantly localized in colon columnar epithelial cells and goblet cells. Additionally, it is found in the stomach, tongue, esophagus, cervix, sweat glands, and prostate [5]. Importantly, in normal physiological conditions, CEACAM5 expression in lung and gastrointestinal epithelial cells is confined to the apical surface of the epithelial cell membrane facing the lumen and remains inaccessible to immune cells.
2. CEACAM5 and cancer
CEACAM5 is predominantly expressed on the surface of epithelial tumor cells, including those associated with colorectal cancer, gastric cancer, pancreatic cancer, gallbladder cancer, lung adenocarcinoma, small cell lung cancer, breast cancer, bladder cancer, ovarian cancer, and more.
2.1. CEACAM5 and colorectal cancer
CEACAM5 is expressed in more than 80% of colorectal cancers (CRC) and has been widely used as a prognostic marker in CRC. It is now clear that patients with elevated preoperative CEACAM5 levels have worse survival outcomes [6]. Studies have shown that forced overexpression of CEACAM5 is associated with anoikis (a form of apoptosis caused by cell-matrix detachment), enhancing cancer cell metastasis [7]. A study conducted by Cai Sanjun’s team has revealed that FBW7 serves as a suppressor of colorectal cancer metastasis by inhibiting the HIF1α/CEACAM5 functional axis [8].
2.2. CEACAM5 and gastric cancer
CEACAM5 is overexpressed in about 90% of gastrointestinal cancers (GC), and this heightened expression has notable consequences. It can disrupt cell polarity and the normal tissue structure, and impede cell differentiation and maturation processes, ultimately culminating in tumor formation [9]. In 2015, Nie Yongzhan’s research team identified CEACAM5 as the antigen of MGd1, a finding that has facilitated the study of CEACAM5 expression in both non-GC and GC tissues. Their investigations unveiled a progressive increase in CEACAM5 expression, ranging from normal gastric mucosa to chronic atrophic gastritis, intestinal metaplasia, atypical hyperplasia, and eventually, gastric carcinoma (GC). Notably, among individuals with gastric precancerous lesions like intestinal metaplasia and dysplasia, those who tested positive for CEACAM5 faced a heightened risk of developing GC compared to CEACAM5-negative individuals.
In addition, CEACAM5 was positively correlated with the depth of invasion of gastric adenocarcinoma. The team believes that CEACAM5 serves as a warning and prognostic biomarker of gastric carcinoma on basis of these results [10]. In 2022, Liu Nian’s team conducted research on the upstream regulatory pathway of CEACAM5 in gastric cancer. Their study illuminated the role of CEACAM5 in promoting gastric cancer cell migration, proliferation, and epithelial-mesenchymal transition (EMT). Additionally, the team identified miR-498 as a key player in controlling the growth of GC cells by downregulating CEACAM5 [11].
2.3. CEACAM5 and NSCLC
CEACAM5 exhibits high expression in approximately 20% of non-small cell lung cancer (NSCLC) adenocarcinoma patients. In 2020, Xinwen Zhang and colleagues from Linyi Central Hospital conducted a study that revealed an increase in CEACAM5 expression in NSCLC tissues and cell lines, as determined by polymerase chain reaction (PCR). This heightened expression was significantly correlated with tumor stage, lymphatic invasion, and histological grade in a cohort of 87 patients, as analyzed through immunohistochemistry (IHC). Moreover, in vitro experiments conducted in this study shed light on the role of CEACAM5 in stimulating NSCLC cell proliferation and migration, which it accomplishes by inhibiting the p38-SMAD2/3 signaling pathway [9].
3. What is the research progress of clinical pipelines targeting CEACAM5?
In terms of clinical development, researchers are developing diverse biotherapeutics targeting CEACAM5, including ADCs, bispecific antibodies, and chimeric antigen receptor T cell (CAR-T) therapies. A majority of these investigations have primarily focused on colorectal cancer and other gastrointestinal malignancies. Preliminary statistics suggest that there are currently approximately 25 CEACAM5-targeted therapies in various stages of preclinical and clinical development. This includes a notable roster of 12 monoclonal antibodies, 5 ADCs, 3 bispecific antibodies, and 5 CAR-T therapies. Noteworthy advancements in this field are highlighted by several therapies advancing to the clinical stage [12].
3.1. Tusamitamab ravtansine
Tusamitamab ravtansine is a new antibody drug conjugate (ADC) introduced by Sanofi from ImmunoGen. This ADC comprises an anti-CEACAM5 humanized monoclonal antibody, coupled with DM4 via a cleavable linker (SPDB) developed by ImmunoGen. DM4 is a cytotoxic maytansinoid alkaloid that specifically targets tumor cells expressing CEACAM5. Currently, tusamitamab ravtansine is in the clinical Phase III stage, with its primary indication being non-small cell lung cancer (NSCLC).
A Phase II clinical trial, known as CARMEN-LC05, is underway to assess the effectiveness and safety of tusamitamab ravtansine in various combination therapies for patients dealing with advanced or metastatic non-squamous NSCLC. These combinations include double therapy with Keytruda, triple therapy with Keytruda and platinum-based chemotherapy, and quadruple therapy involving Keytruda, platinum-based chemotherapy, and pemetrexed. In all treatment groups, patients received intravenous tusamitamab ravtansine at doses of either 150 mg/m2 or 170 mg/m2 every three weeks. The results of this trial are promising, with an overall response rate (ORR) of 52% and a median treatment duration of 24.3 weeks. Additionally, 36% of patients exhibited stable disease, while only 12% experienced disease progression, resulting in an impressive disease control rate of 88%. Notably, positive responses were observed across all PD-L1 expression level groups, as well as in patients with medium/high CEACAM5 expression levels. The combination therapy, particularly the double combination group, demonstrated outstanding efficacy [12].
3.2. Labetuzumab govitecan
Labetuzumab govitecan (IMMU-130), an ADC drug developed by Immunomedics, comprises an anti-CEACAM5 monoclonal antibody linked to SN-38 through a pH-sensitive linker. SN-38 is the active metabolite of the antineoplastic drug irinotecan. Currently, this drug is in the clinical Phase II stage, with its primary indications centered around colorectal cancer.
A noteworthy phase 1/2 trial study, published in the journal “J Clin Oncol” on August 17, 2017, evaluated the impact of labetuzumab govitecan in treating relapsed or refractory metastatic colorectal cancer (mCRC). The study enrolled 86 patients who had refractory colorectal cancer and had undergone an average of five prior treatments. These patients were subjected to labetuzumab govitecan treatment at doses of 8 and 10 mg/kg once weekly or 4 and 6 mg/kg twice weekly during weeks 1 and 2 of a 3-week cycle. The results revealed that 38% of patients experienced various degrees of tumor shrinkage. Notably, one patient achieved a partial response that persisted for over 2 years, while 42 patients achieved stable disease. The median overall survival was recorded at 6.9 months. These findings strongly suggest that labetuzumab govitecan monotherapy exhibits a manageable safety profile and demonstrates therapeutic activity in the treatment of colorectal cancer [13].
3.3. cibisatamab
Cibisatamb, a CEACAM5xCD3 bispecific antibody developed by Roche, as a T cell bispecific antibody that binds to CEACAM5 on cancer cells and CD3 on T cells. This unique structure enables one “arm” of Cibisatamb to attach to CEACAM5 while the other engages with T cells, thereby activating them and prompting an immune response against tumors. Cibisatamb features a single binding site for CD3ε chain and two CEA binding sites on T cells. Currently, it is undergoing clinical Phase II research, with its primary focus on colorectal cancer.
Roche has adopted a strategy that does not involve using cibisatamab as a single agent in clinical development. Instead, there are two ongoing clinical Phase I/II trials. The first trial (NCT04826003) combines cibisatamb with the FAP/4-1BB dual antibody RO7122290, which serves as a costimulatory dual antibody. The second trial (NCT03866239) combines cibisatamab with the PD-L1 monoclonal antibody atezolizumab, acting as a T cell modulator. These studies target patients with previously treated metastatic, microsatellite-stable colorectal adenocarcinoma expressing high levels of CEACAM5. Currently, both trials are actively recruiting participants [12].
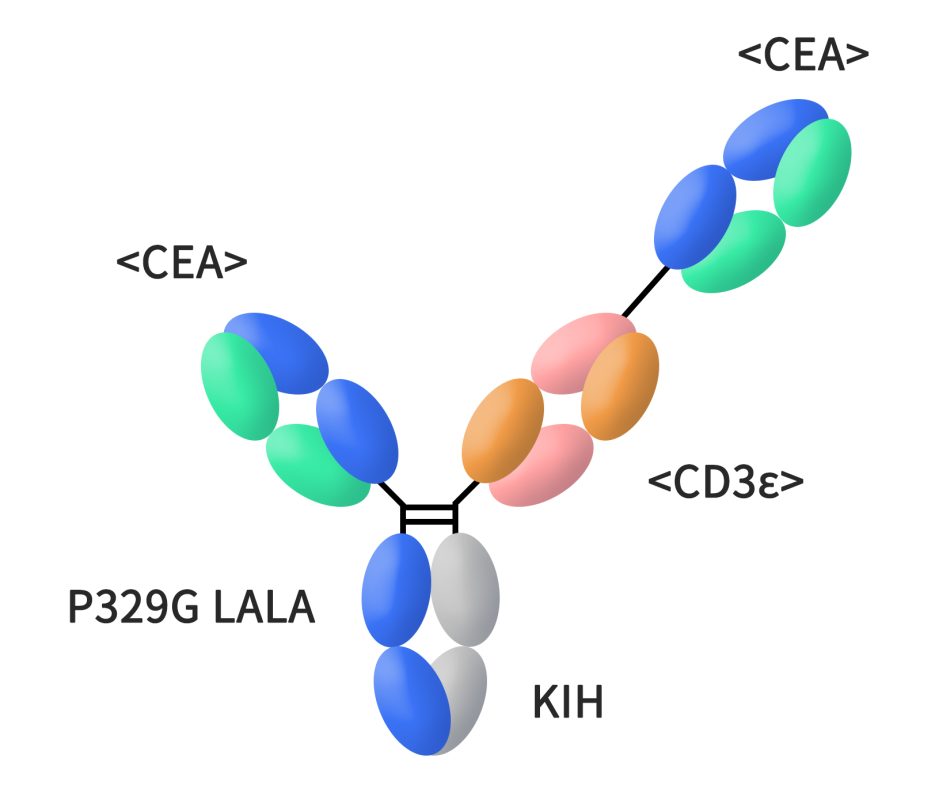
Figure 2. The structure of cibisatamab
3.4. MG7-CART
MG7 is a glycosylated CEACAM5 protein, recognized as a gastric cancer-related antigen with remarkable specificity and sensitivity. MG7-CART represents an innovative chimeric antigen receptor (CAR) therapy that has been engineered with anti-MG7 antibodies. This therapy has been developed by Genetech and has demonstrated promising results.
Genetech’s presentation at the 2017 AACR meeting highlighted the significant efficacy of MG7-CART in inhibiting the growth of patient-derived xenograft (PDX) tumors within a gastric cancer model, resulting in the elimination of 60% of tumors in mice. This groundbreaking outcome underscores the potential of MG7-CART in cancer treatment.
At present, MG7-CART is advancing through clinical Phase II research, with its primary indications centered around colorectal cancer and gastric cancer [13].
4. What products and services can DIMA supply?
DIMA is a forward-thinking biotechnology company specializing in offering preclinical R&D products and services designed for druggable targets. The company boasts a range of cutting-edge platforms, including innovative functional membrane protein preparation, single B cell lead antibody discovery, and antibody engineering and functional verification platforms. DIMA’s primary objective is to facilitate pharmaceutical companies in establishing monoclonal antibody platforms and streamlining lead molecule screening, thereby expediting the progression of clinical pipelines.
Specifically concerning the CEACAM5 target, DIMA offers a comprehensive array of products and services tailored to meet the demands of CEACAM5 antibody drug-related research. These offerings encompass active proteins, rabbit monoclonal antibodies, and reference antibodies. Furthermore, DIMA has undertaken the development of CAR (Chimeric Antigen Receptor) molecules and has successfully conducted in vitro verification experiments on these CAR molecules
4.1. CEACAM5 active protein
Human CEACAM5 Protein, His Tag (PME100071)
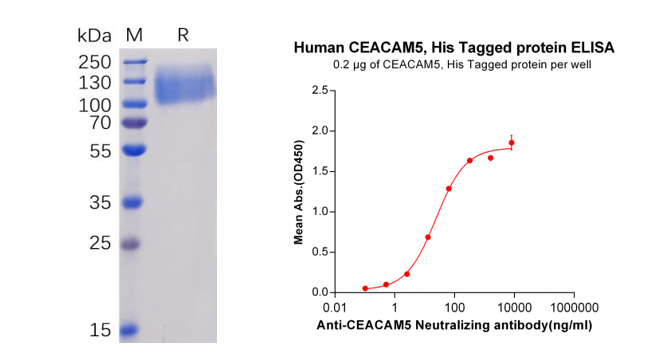
Figure 3. Validation data of purified Human CEACAM5 Protein, His Tag (PME100071). Human CEACAM5 Protein, His Tag on SDS-PAGE (left); CEACAM5 Protein (PME100071) can bind Anti-CEACAM5 Antibody (BME100035) in a linear range of 2.56-320 ng/ml (right).
More proteins:
Mouse CEACAM5 Protein, His Tag (PME-M100060)
4.2. CEACAM5 rabbit monoclonal antibody
Anti-CEACAM5 antibody(DM120); Rabbit mAb (DME100120)
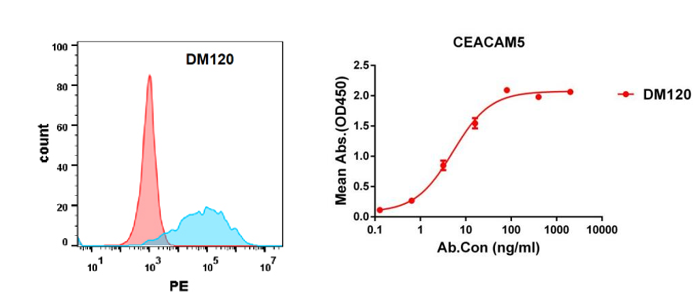
Figure 4. Validation data of Anti-CEACAM5 antibody(DM120) Rabbit mAb (DME100120). Flow cytometry analysis with Anti-CEACAM5 (DM120) on Expi293 cells transfected with human CEACAM5(Blue) or irrelevant protein (Red) (left). Anti-CEACAM5 antibody(DM120) (DME100120) can bind His tagged protein (PME100071) in a linear range of 0.1-50 ng/ml (right).
More rabbit monoclonal antibody:
Anti-CEACAM5 antibody(DM122); Rabbit mAb (DME100122)
Anti-CEACAM5 antibody(DM121); Rabbit mAb (DME100121)
Furthermore, DIMA has effectively generated a single B cell seed library for CEACAM5. This innovative resource accelerates the screening process for potential CEACAM5 lead antibody molecules, with results available in as little as 20 days. For additional details and information on DIMA’s single B cell seed library, please Find a link to know more information about DIMA single B cell seed library>>
4.3. CEACAM5 reference antibody
Anti-CEACAM5 (labetuzumab biosimilar) mAb (BME100035)
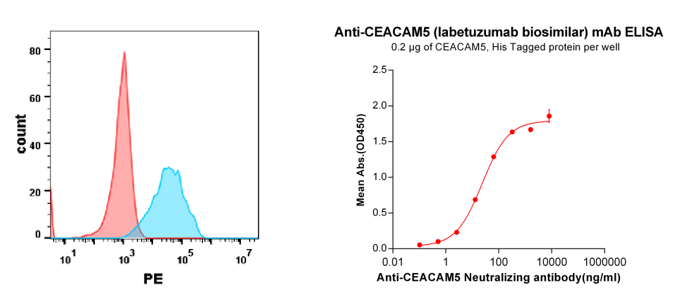
Figure 5. Validation data of Anti-CEACAM5 (labetuzumab biosimilar) mAb (BME100035); Flow cytometry analysis with Anti-CEACAM5 (labetuzumab) on Expi293 cells transfected with human CEACAM5 (Blue) or Expi293 transfected with irrelevant protein(Red) (left). His tagged protein (PME100071) can bind Anti-CEACAM5 Neutralizing antibody(BME100035) in a linear range of 2.56-320 ng/ml (right).
More benchmark reference antibodies:
Anti-CEACAM5 (Chongqing Precision biosimilar) mAb (BME100182)
4.4. CEACAM5 Antibody Humanization: Case Demonstrations
The CEACAM5 antibody’s affinity was entirely compromised during its humanization using conventional computer-aided design. However, through screening with a library of humanized antibodies created by DIMA, a clone with significantly greater affinity than the chimeric antibody was successfully identified.
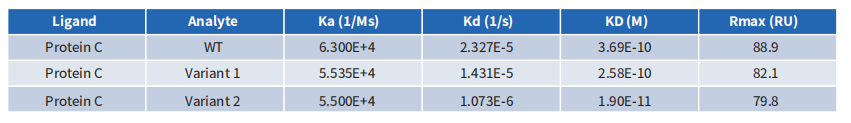
4.5. 24-hour in vitro killing test of CEACAM5 CAR T cells
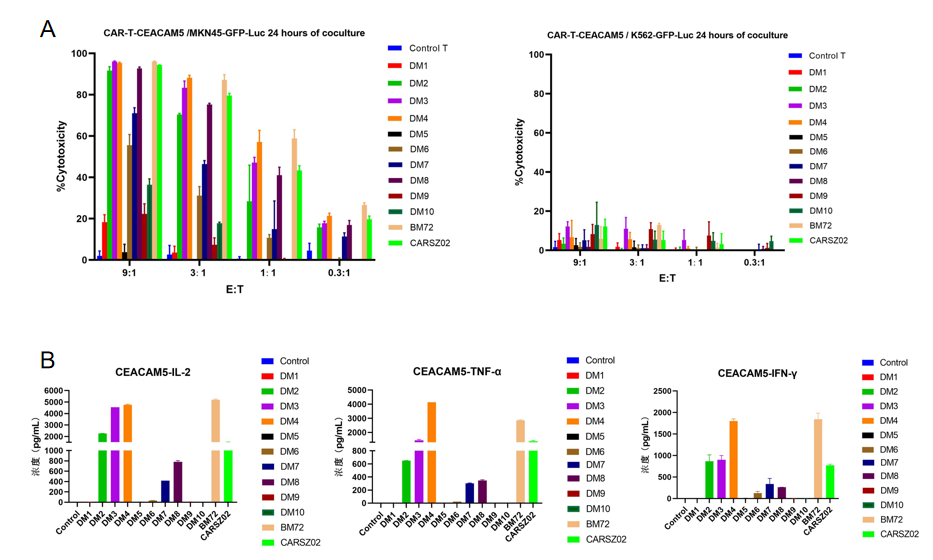
Figure 6. 24-hour in vitro killing test of CEACAM5 CAR T cells carrying humanized scFv constructs developed by DIMA tech. The results show that most of these CAR T cells effectively killed MKN-45 cells (naturally expressing CEACAM5) while not killing K562 cells (negative control). IL-2, TNF-α, and IFN-γ were detected 24 hours after the killing experiment at the effect target ratio of 3:1.