Figure 2: Quality analysis of purified human BCMA protein, Fc-tagged. A. Human BCMA, Fc-tagged on SDS-PAGE under reducing condition. B. ELISA plate pre-coated by 2 μg/ml (100 μl/well) Human BAFF, hFc tagged protein can bind Human BCMA, Fc- tagged protein in a linear range of 0.03-15.625 ng/ml. C. ELISA plate pre-coated by 2 μg/ml (100 μl/well) Human BCMA, Fc-tagged protein can bind Anti-BCMA (huC11D5.3) (Its variable region was used to construct scFv portion of CAR-T Idecabtagene vicleucel (bb2121). ) in a linear range of 3.71-22.29 ng/ml.
Antibody Request Form
Development of anti-BCMA therapeutic mAbs for CAR-T application
Our pre-selected lead mAb molecules have been successfully used by different pharmaceutical companies for their drug development. Here is one of our case studies for the anti-BCMA therapeutic lead mAb molecule development (Figure 1).
We have prepared a large amount of human BCMA recombinant proteins as the antigen for immunization. The purities and activities of the proteins were validated by SDS-PAGE and different binding assays (Figure 2) before immunization.
After immunization, around 3~5 X10^8 B cell pools were isolated from the immunized rabbit and quickly frozen in liquid nitrogen as our B-cell seed library for human BCMA target. From 4ml of rabbit whole blood, we identified 70 ELISA positive B cell clones, out of which 13 worked for the flow application and were picked up for the downstream mAb cloning and sequencing. After Cand epitope comparison, 5 new clones were identified with unique CDR sequences comparing to the Bluebird anti-BCMA huC11D5.3 clone (Figure 3 & 4). All of them have shown comparable tumor cell killing efficacy with the Bluebird anti-BCMA huC11D5.3 clone in the CAR-T application after humanization (Figure 5). One has been picked up for an Investigator Initiated trial (IIT). The preliminary data is encouraging at this moment.
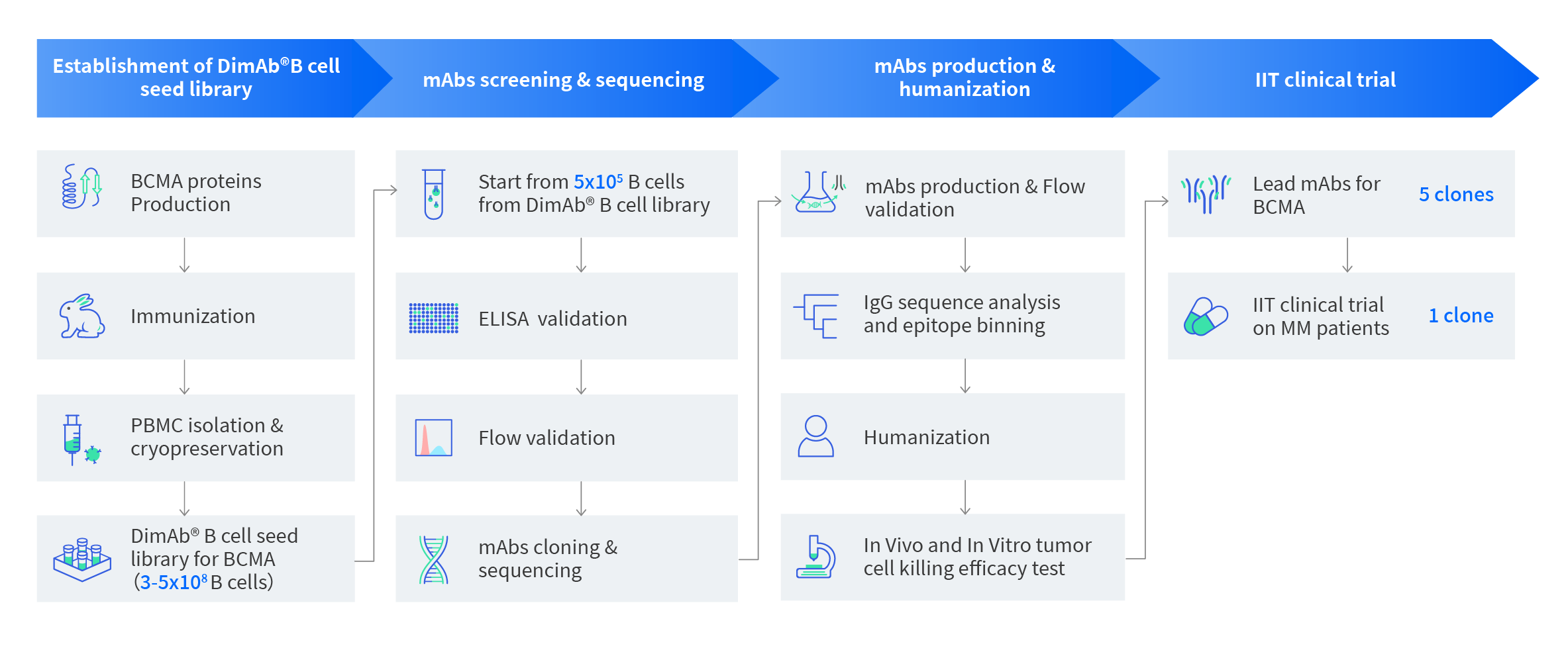
Figure 1: Workflow for BCMA mAb lead molecules project. From 4ml of rabbit whole blood, we identified 5 lead mAbs molecules for human BCMA targets with verified functional data and antibody sequences. We also created a DIMA mAb B cell seed library for the human BCMA target which presumably contains 10 thousands Flow positive BCMA binders providing a good resource for additional screening.
Functional BCMA protein used as immunogen
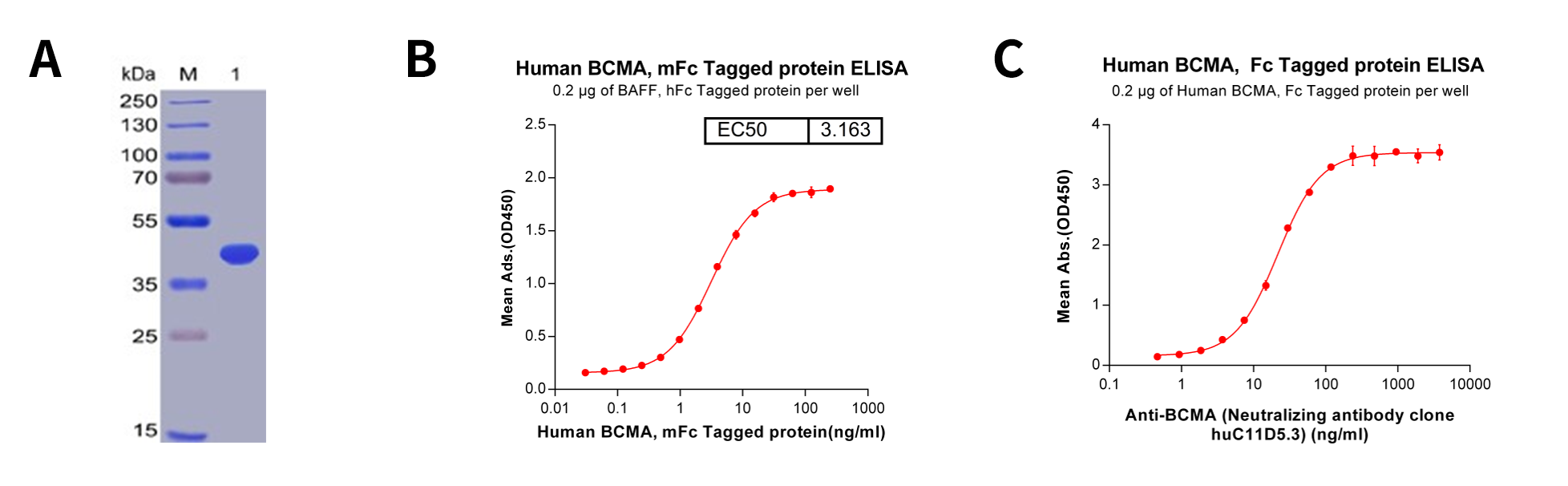
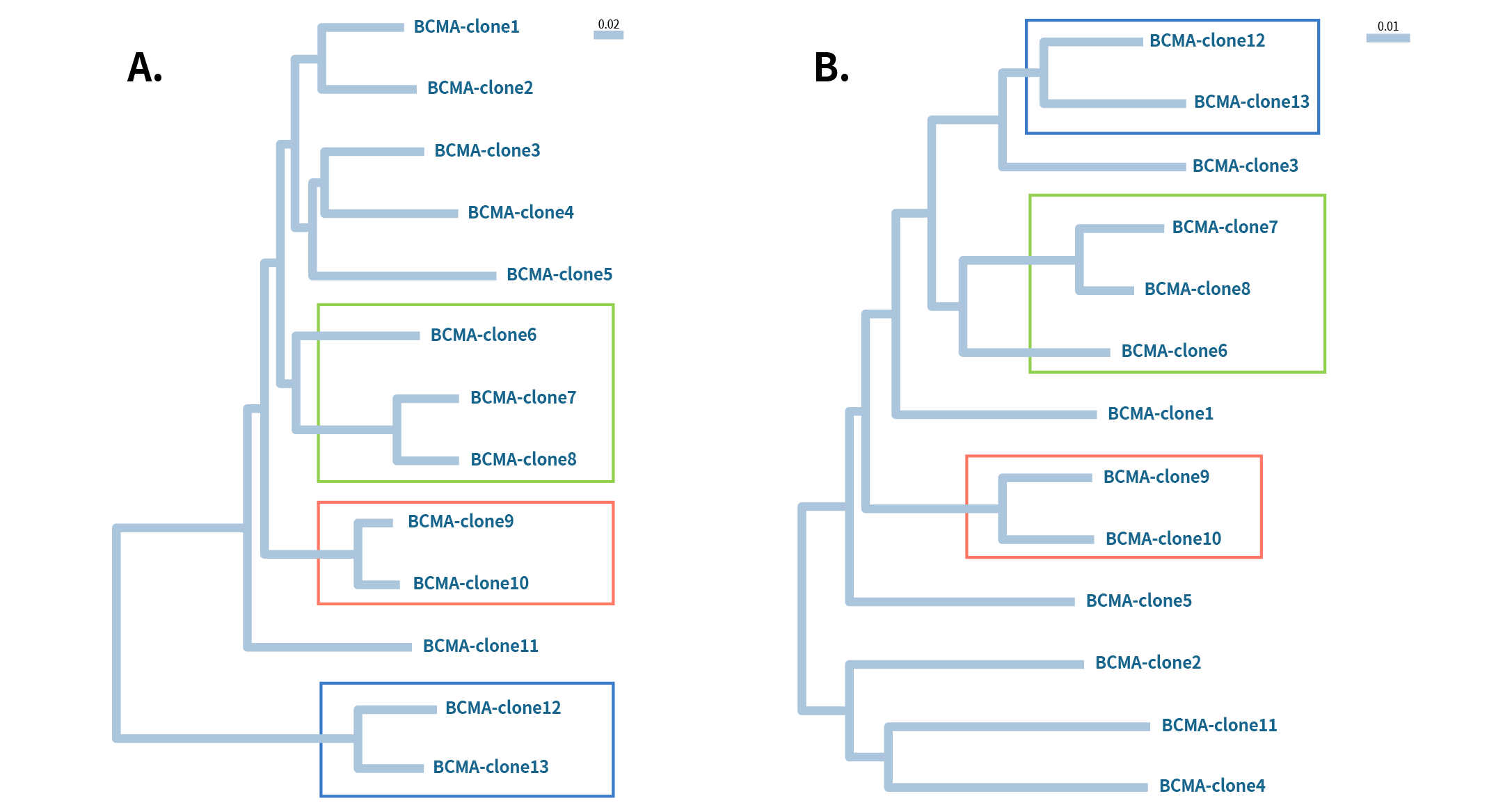
Figure 3: Phylogenetic analysis of 13 different Anti-BCMA mAb clones A) heavy chain and B) Light chain. All these clones work for flow application. The boxed regions indicate heavy and light chains of the same clone come from the same lineage group.
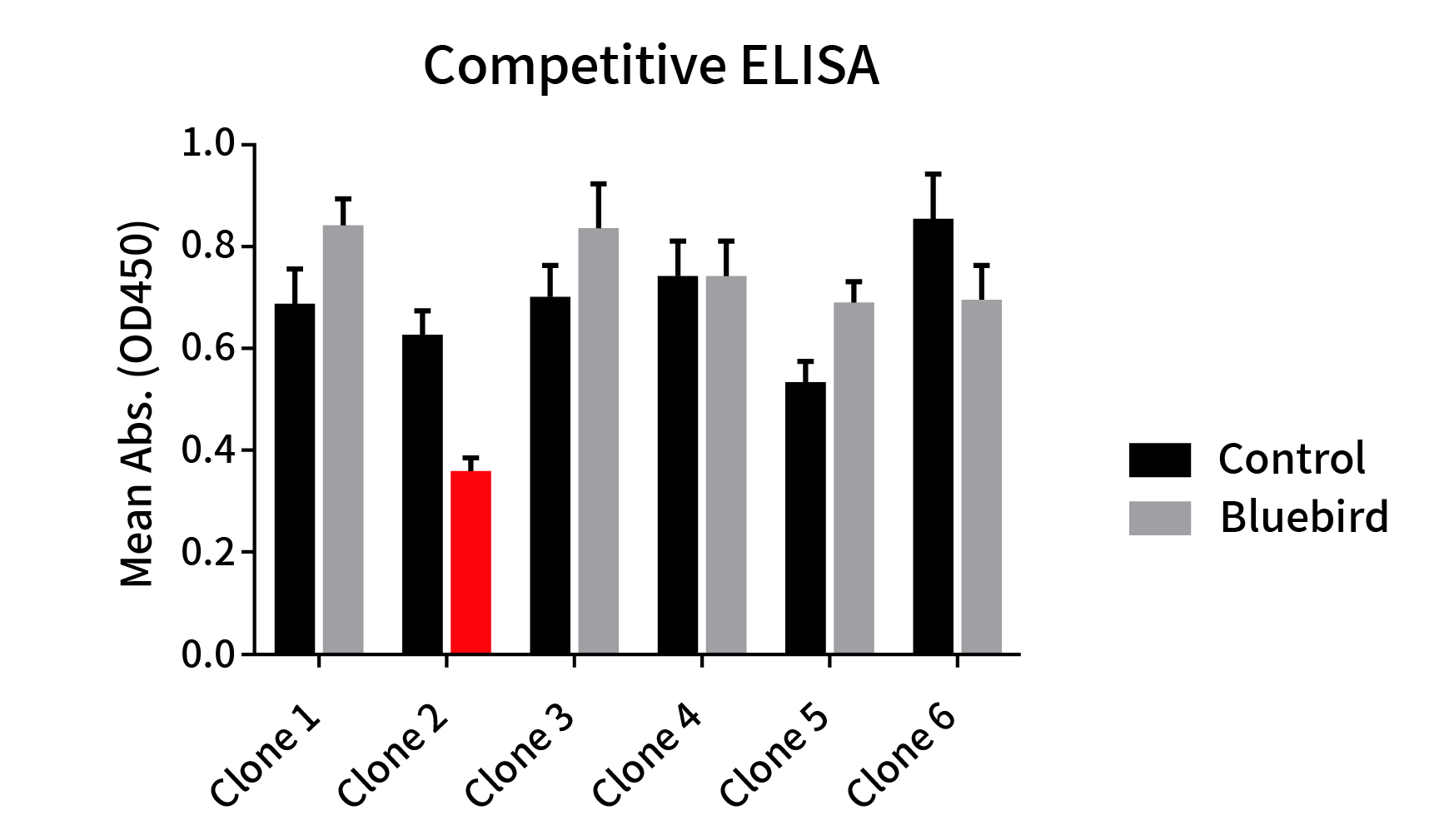
Figure 4: Epitope comparison between different anti-BCMA mAb clones and anti-BCMA huC11D5.3 clone (Bluebird bb2121). ELISA plate was coated with recombinant BCMA-hFc fusion protein, followed by pre-blocking with huC11D5.3 antibody (Grey bar) or rabbit control IgG (Black bar) and then different rabbit mAbs antibodies were added to check the competitive inhibition of huC11D5.3. One clone exhibits the strongest inhibition (Red bar). This data indicated that one clone binds to the same epitope as bb2121.
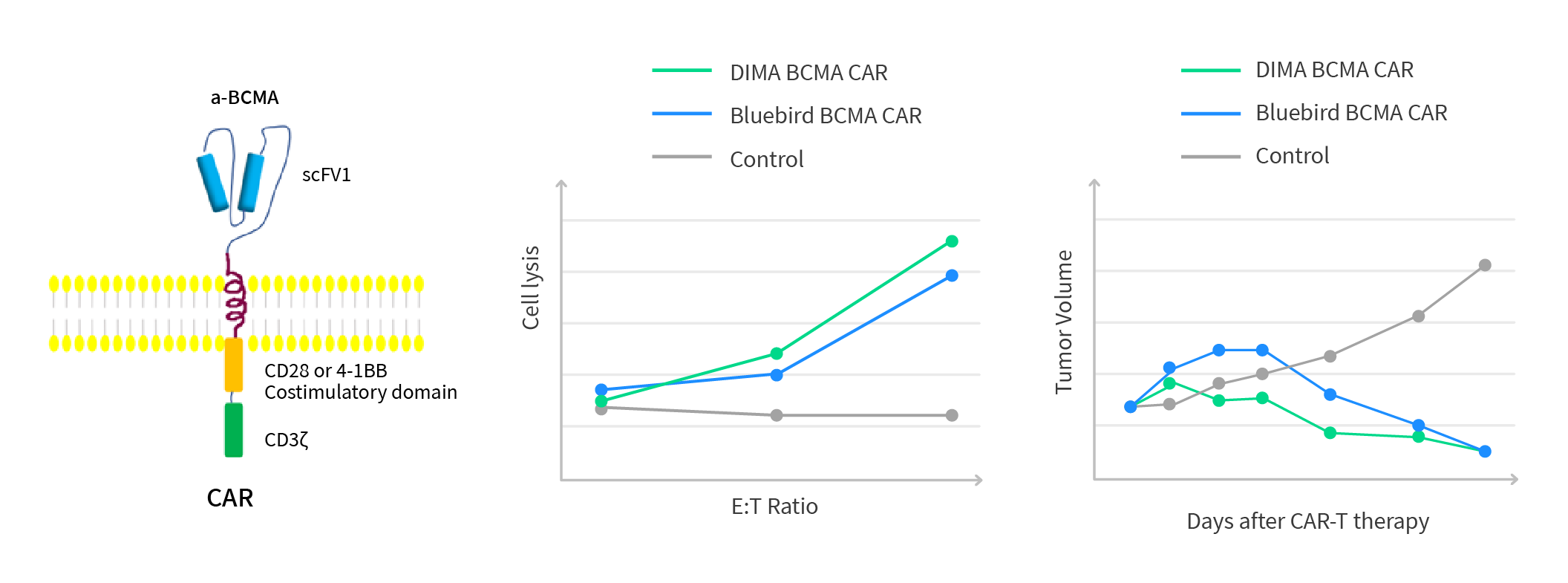
Figure 5: In-vivo testing of humanized anti-BCMA lead mAbs molecules. The preliminary tumor cell killing efficacy testing data is proprietary. It indicated that our 5 humanized CARs are comparable or better than BMK.
