When it comes to weight loss medications, one cannot overlook the remarkable success of Semaglutide (GLP-1 weight loss drug), which has propelled the Danish biopharmaceutical company, Novo Nordisk, to new heights. According to Novo Nordisk’s financial report for 2023, Semaglutide sales reached $21.2 billion in 2023. On March 7th, Novo Nordisk’s stock price surged by over 10%, briefly exceeding a market capitalization of $610 billion. Currently, Novo Nordisk’s weight loss drug Wegovy remains on the drug shortage list of the US Food and Drug Administration (FDA). The vast market for weight loss drugs has attracted the attention of the entire industry, with a plethora of new mechanism drugs, including ActRIIA/B antibodies, NLRP3 inhibitors, and GPR75. In this article, we will focus on the emerging weight loss target, GPR75.
1. GPR75 and the GPCR Family
G protein-coupled receptor 75 (GPR75) is a member of the G protein-coupled receptor (GPCR) family, initially discovered by Tarttelin et al. in 1999 [1]. GPCRs are a class of membrane proteins widely distributed on the cell surface, characterized by seven hydrophobic transmembrane segments, making them the largest and most diverse receptor protein family in the human body. The typical structure of the GPCR superfamily consists of three parts: an extracellular amino-terminal, seven hydrophobic transmembrane (7TM) segments, and an intracellular carboxy-terminal. When various extracellular ligands (such as hormones, chemokines, neurotransmitters, autocrine hormones, and enzymes) bind to GPCRs, it induces conformational changes in the receptor. These conformational changes further lead to the interaction of GPCRs with heterotrimeric G proteins, resulting in numerous cellular physiological functions [2].
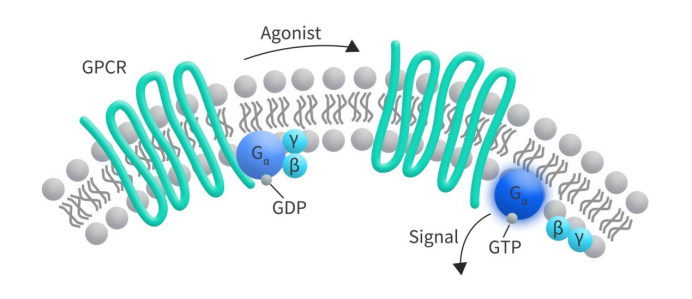
Figure 1. The structure of GPCR [2]
To date, approximately 800 members of this family have been identified, with more than half being olfactory receptors. Based on sequence similarity, GPCRs can be divided into six major classes: class A (rhodopsin-like family), class B (Methuselah-like, adhesion, and secretin-like receptor family), class C (metabotropic glutamate receptor family), class D (fungal mating pheromone receptors), class E (cAMP receptor family), and class F (frizzled/smoothened receptor family). GPR75 belongs to the rhodopsin-like family and is a unique member of the GPCR family. GPR75 is a protein consisting of 540 amino acids, with only two exons, located on human chromosome 2p16. The first exon of GPR75 contains a non-coding sequence, while the second exon of GPR75 contains the entire coding region of GPR75, which is not similar to any other gene or transcript [3]. The highest expression of the GPR75 gene is in the brain; however, recent studies have suggested that GPR75 is expressed in most human tissues, including the brain, heart, kidneys, and prostate.
2. GPR75 Ligands and Signaling Pathways
While GPR75 has long been considered an orphan receptor, ongoing research has gradually revealed its ligands. To date, three ligands for GPR75 have been identified, including RANTES, CCL5, and 20-HETE, with 20-Hydroxyeicosatetraenoic acid (20-HETE) being the most extensively studied.
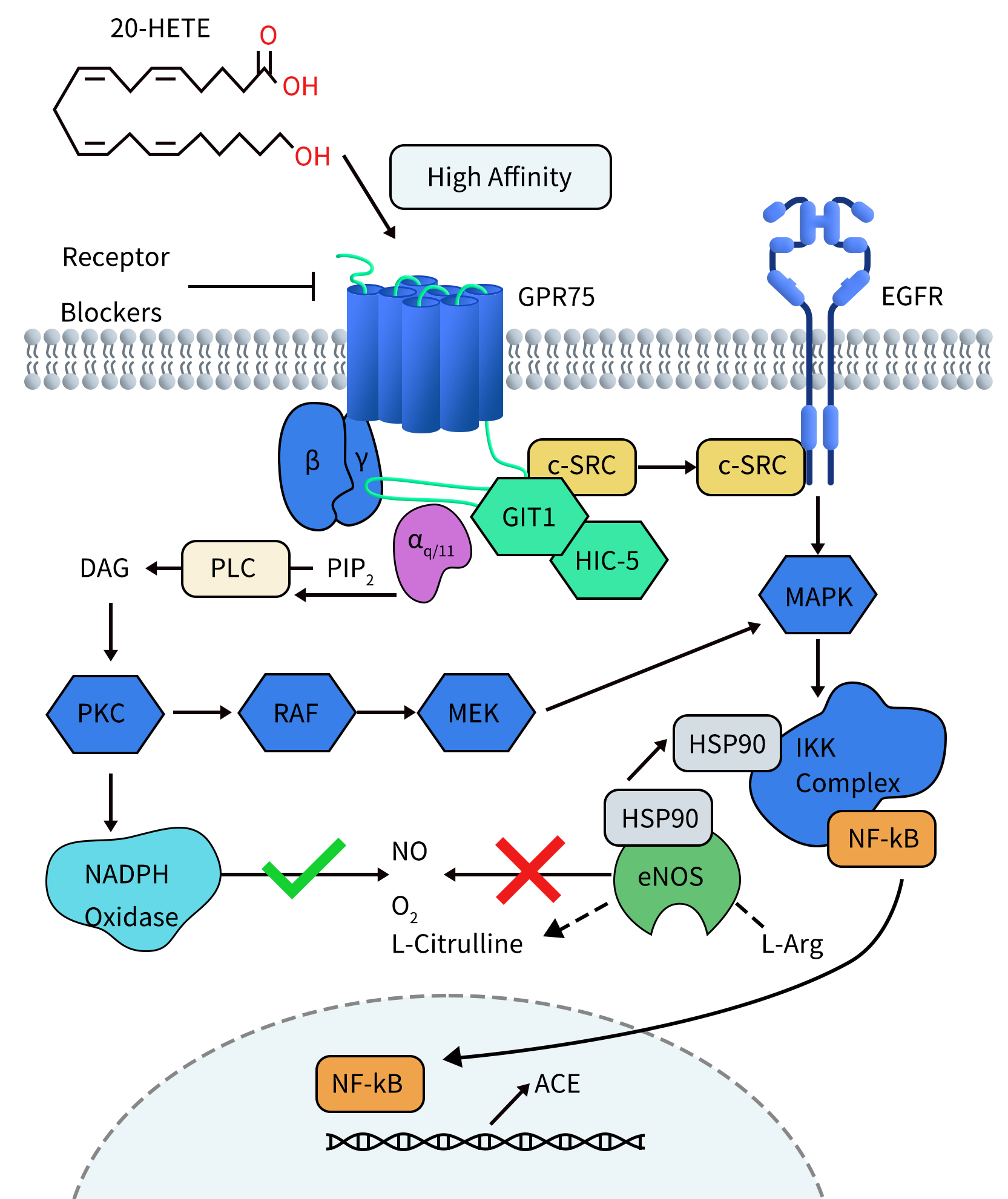
In studies investigating the pro-inflammatory and hypertensive effects of 20-HETE, Victor Garcia et al. found that 20-HETE can bind to its receptor GPR75. Activation of GPR75 leads to Gαq/11-driven signal transduction, subsequently activating the epidermal growth factor receptor (EGFR) through a GIT1–/c-SRC-dependent pathway, thereby stimulating the activation of MAPK and IKK complexes. Activated IKK induces two events: firstly, IKK triggers the translocation of NF-KB to the cell nucleus. Within the nucleus, NF-KB binds to the promoter region of the angiotensin-converting enzyme (ACE) gene, leading to increased ACE transcription. Secondly, activated IKK promotes the uncoupling of endothelial nitric oxide synthase (eNOS) by recruiting the chaperone protein HSP90, resulting in decreased bioavailability of nitric oxide (NO). Furthermore, signaling through the 20-HETE receptor promotes mechanisms driven by protein kinase C (PKC) activation, including increased reactive oxygen species (ROS) generation derived from NADPH oxidase. These signaling events contribute to endothelial dysfunction and initiate pro-inflammatory pathways, leading to conditions such as diabetes, obesity, endothelial dysfunction, cell proliferation, hypertension, and cardiovascular diseases.
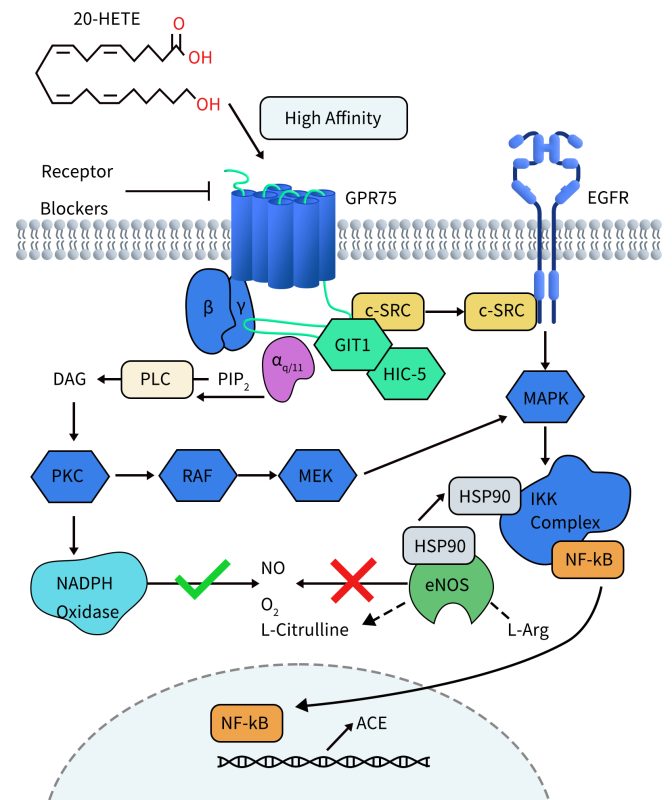
Figure 2. 20-HETE Receptor (GPR75) Signaling in Endothelial Cells [4]
Additionally, Victor Garcia et al. demonstrated in a 20-HETE-dependent hypertension mouse model that knocking down GPR75 prevented the increase in blood pressure, as well as the upregulation of ACE expression, endothelial dysfunction, smooth muscle contractility, and vascular remodeling mediated by 20-HETE [5].
3. GPR75 and Diseases
GPR75 is a novel member of the GPCR family and plays a significant role in various diseases such as obesity, cancer, and metabolic syndrome. As mentioned earlier, GPR75 has three ligands: RANTES, CCL5, and 20-HETE, each of which triggers multiple signaling pathways. Due to the binding of 20-HETE to GPR75, the PKC/PLC, C-src, MAPK, NF-κB, and AKT signaling pathways are activated, leading to inflammation, diabetes, vascular dysfunction remodeling, hypertension, and malignant cell transformation. 20-HETE can increase the invasiveness of non-small cell lung cancer cells and the metastatic potential of prostate cancer cells. Studies have shown that blocking the 20-HETE pathway can reduce the risk of brain cancer, breast cancer, and renal cancer, as well as the migration and invasion of triple-negative breast cancer cells. Generally, 20-HETE enhances the metastatic properties of cancer cells through GPR75. Additionally, 20-HETE regulates actin polymerization by increasing the expression of stress fibers and focal adhesion kinase, thereby affecting cell adhesion and polarization, which in turn affects cell migration and invasion [6].
The emergence of GPR75 as a promising target for obesity treatment is based on a study published by Regeneron in the Science in July 2021. This study conducted whole-exome sequencing on 645,000 individuals from the UK, US, and Mexico to analyze the correlation between rare genetic mutations and body mass index (BMI). The researchers identified 16 nonsynonymous gene mutations associated with BMI. Among them, they found that the truncation mutation of GPR75 had an occurrence rate of 0.04%. Heterozygous mutation carriers had a BMI that was 1.8 kg/m² lower, a weight that was 5.4 kg lighter, and a 54% lower probability of obesity. In vivo validation experiments in mice conducted as part of this study showed that GPR75 knockout mice had lower body weight, lower blood glucose levels, and lower insulin levels [3].
4. Clinical Research Progress on GPR75
As of now, there are no GPR75-targeted drugs in clinical stages. In July 2021, following the publication of Regeneron’s research, AstraZeneca and Regeneron announced that they had entered into a collaboration to research, develop, and commercialize small molecule compounds targeting GPR75, which could potentially be used to treat obesity and related diseases. Currently, Regeneron is developing various therapies for GPR75, including siRNA, small molecules, and antibodies, through internal and external collaborations. In 2023, ConfometRx disclosed its GPR75 pipeline on its corporate website, which is currently in the lead optimization stage. In October 2023, Guangzhou Bebetter Med announced the first patent for a small molecule targeting GPR75. This molecule is an indirect inhibitor of GPR75, capable of overcoming GLP-1 resistance without causing appetite side effects. At the time of disclosure, the molecule was in the CMC stage, with an expected IND submission in 2023.
5. DIMA’s GPR75-Related Products and Services
Dima Biotech is a biotechnology company dedicated to the preclinical research and development of drug targets. Currently, we offer a range of products and services related to GPR75 to support drug development targeting this protein. Our products include full-length active proteins, ECD active proteins, and flow cytometry-validated monoclonal antibodies targeting GPR75. Additionally, we provide various custom antibody services, including antibody humanization and affinity maturation, to meet the diverse needs of our customers. In order to expedite the development of GPR75-based therapies, we have also established a GPR75 target single B cell seed library, from which lead antibody molecules can be obtained in as little as 28 days. We are committed to providing high-quality products and services to assist our customers in achieving success in the field of drug development.
- Full-length recombinant protein
Human GPR75 full-length protein-synthetic nanodisc (FLP100031)
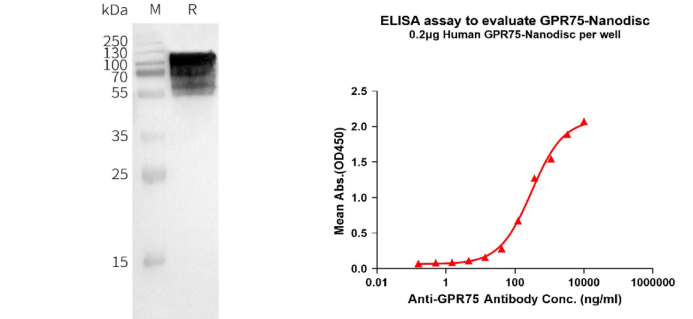
Figure 3. The validated data of FLP100031. WB analysis of Human GPR75-Nanodisc with anti-Flag monoclonal antibody at 1/5000 dilution, followed by Goat Anti-Rabbit IgG HRP at 1/5000 dilution (left). ELISA plates were pre-coated with Flag Tag GPR75-Nanodisc (0.2μg/per well). The EC50 for anti-GPR75 monoclonal antibody binding with GPR75-Nanodisc is 303.6ng/ml. (right).
- ECD recombinant protein
Human GPR75 Protein, hFc Tag (PME100704)
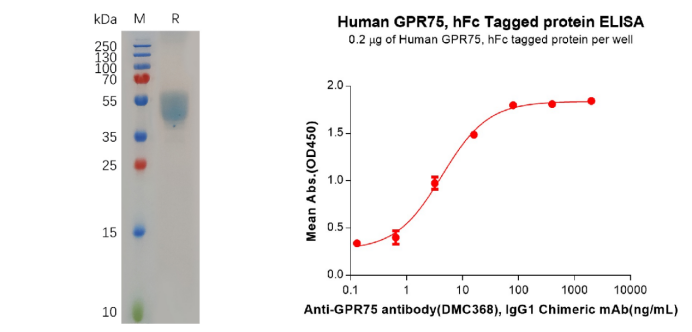
Figure 4. The validated data of PME100704. The purity of the protein is greater than 95% as determined by SDS-PAGE and Coomassie blue staining (left). ELISA plate pre-coated by 2 μg/mL (100 μL/well) Human GPR75 Protein, hFc Tag can Anti-GPR75 antibody(DMC368), IgG1 Chimeric mAb in a linear range of 0.64–16 ng/mL.
- FC-validated antibody
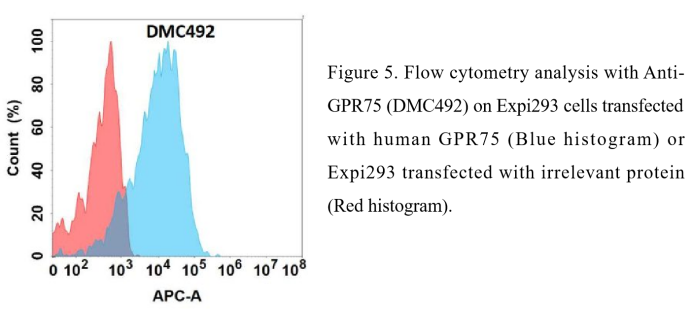
Anti-GPR75 antibody(DMC492); IgG1 Chimeric mAb (DMC100492)
Reference:
[1]Tarttelin EE, Kirschner LS, Bellingham J, et al Cloning and characterization of a novel orphan G-protein-coupled receptor localized to human chromosome 2p16. Biochem Biophys Res Commun. 1999 Jun 24;260(1):174-80.
[2]Kawasawa Y, McKenzie LM, Hill DP, et al. RIKEN GER Group; GSL Members. G protein-coupled receptor genes in the FANTOM2 database. Genome Res. 2003 Jun;13(6B):1466-77.
[3]Akbari P, Gilani A, Sosina O, et al. Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science. 2021 Jul 2;373(6550):eabf8683.
[4]Froogh G, Garcia V, Laniado Schwartzman M. The CYP/20-HETE/GPR75 axis in hypertension. Adv Pharmacol. 2022;94:1-25.
[5]Garcia V, Gilani A, Shkolnik B, et al. 20-HETE Signals Through G-Protein-Coupled Receptor GPR75 (Gq) to Affect Vascular Function and Trigger Hypertension. Circ Res. 2017 May 26;120(11):1776-1788.
Cárdenas S, Colombero C, Panelo L, et al. GPR75 receptor mediates 20-HETE-signaling and metastatic features of androgen-insensitive prostate cancer cells. Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Feb;1865(2):158573.