In December 2023, GlaxoSmithKline (“GSK”) invested $1.71 billion to obtain the global exclusive rights for the development, production, and commercialization of HS-20093 from HANSON PHARAM (excluding mainland China, Hong Kong, Macau, and Taiwan). HS-20093 is a novel B7-H3 targeted antibody-drug conjugate (ADC) developed independently by HANSON PHARAM. Similarly, in April 2024, Macrogenics’ stock price surged by 30% after announcing the latest data from the TAMARACK Phase II clinical trial of vobramitamab duocarmazine (MGC-018), a new B7-H3 ADC therapy for castration-resistant prostate cancer. As a result, B7-H3 is considered a highly promising ADC target. But what exactly is B7-H3? What role does it play in the development and progression of tumors? Apart from ADC, what other therapies target B7-H3, and which pharmaceutical companies are investing in this target?
1. B7-H3 Structure and Distribution
B7 homolog 3 (B7-H3), also known as CD276, belongs to the B7 family of immune regulatory proteins. Other members of the B7 family include: B7-1 (CD80), B7-2 (CD86), B7-DC (PD-L2 or CD273), B7-H1 (PD-L1 or CD274), B7-H2 (ICOSLG), B7-H4 (VTCN1), B7-H5 (VISTA), B7-H6 (NCR3LG1), and B7-H7 (HHLA2) [1]. The B7 family has been demonstrated to act as co-stimulatory or co-inhibitory molecules in T cell activation, with their ligands and receptors playing crucial roles in adaptive immune responses and malignant tumors.
B7-H3 was first cloned from a human dendritic cell (DC) cDNA library in 2001 [2]. It shares 20-27% amino acid identity with other members of the B7 family. The amino acid sequence of B7-H3 is 88% homologous between mice and humans [3]. Despite this similarity, human-specific antibodies against B7-H3 do not cross-react with endogenous mouse B7-H3 expressed in mouse cells [4]. The genes encoding the B7-H3 protein in mice and humans are located on chromosome 9 and chromosome 15q24, respectively.
The human B7-H3 protein exists in two subtypes: transmembrane and soluble. Transmembrane B7-H3 is a type I transmembrane protein composed of 316 amino acids, with a molecular weight ranging from 45-66 kDa. It consists of extracellular, transmembrane, and short intracellular domains. Based on its extracellular domain, B7-H3 has two subtypes. The first is 4IgB7-H3 (4Ig VCVC), which contains two pairs of identical IgV-like and IgC-like domains. The second is 2IgB7-H3 (2Ig VC), which contains a single pair of IgV-like and IgC-like domains. Among these, 4IgB7-H3 is the predominant subtype in humans. It’s worth noting that in mice, B7-H3 is expressed only as the 2IgB7-H3 subtype. Soluble B7-H3 is generated by cleavage from membrane-bound B7-H3 by matrix metalloproteinases (MMPs). Soluble B7-H3 includes the receptor-binding domain of B7-H3 and is an active form that binds to receptors. The addition of MMP inhibitors can prevent the release of B7-H3 from cells, leading to its accumulation on the cell surface [5]. The main subtype with soluble isoforms is 2IgB7-H3, as the first IgC domain of 4IgB7-H3 generates a new conserved region, which may hinder the release of soluble forms [6].
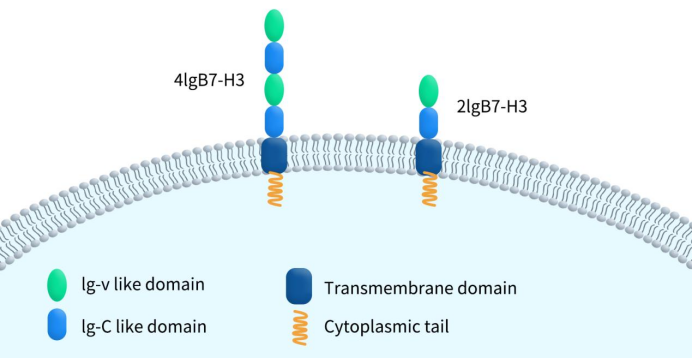
Figure 1. The structure of B7-H3 (transmembrane isoform)
B7-H3 is widely expressed at the RNA level in both lymphoid and non-lymphoid organs. However, expression of the B7-H3 protein is more restricted and is mainly found in activated DCs, monocytes, T cells, B cells, and NK cells [7]. While it is present at low levels in most normal tissues, B7-H3 is highly expressed in various solid tumors, including bladder cancer, breast cancer, cervical cancer, colorectal cancer, esophageal cancer, glioma, kidney cancer, liver cancer, lung cancer, ovarian cancer, pancreatic cancer, and prostate cancer, among others.
2. B7-H3’s Role in Tumor Development
B7-H3 can act as both a co-stimulatory and/or co-inhibitory molecule, depending largely on the immune cells involved and the microenvironmental conditions. It plays a significant role in various processes during tumor development, immune evasion, and is associated with poor prognosis in cancer. B7-H3 influences immune responses and cancer progression through both immunological and non-immunological pathways. Although the receptor for B7-H3 has not been definitively identified, it is speculated that receptors expressed by activated CD4+ and CD8+ T cells may be recognized by antigen-presenting cells (APCs) or tumor cells expressing B7-H3 [8]. Both the 2IgB7-H3 and 4IgB7-H3 subtypes of B7-H3 can inhibit T cell proliferation and downregulate cytokine production [9]. The expression of B7-H3 can suppress the tumor microenvironment by promoting the secretion of IL-10 and TGF-β1. It inhibits the activity of various immune cells, including CD4+ T cells, CD8+ T cells, γδT cells, CAR-T cells, Vδ2T cells, Th17 cells, CD3+ T cells, NK cells, macrophages, neutrophils, dendritic cells, and also suppresses the secretion of IFN-γ, IL-2, perforin, and granzyme B.
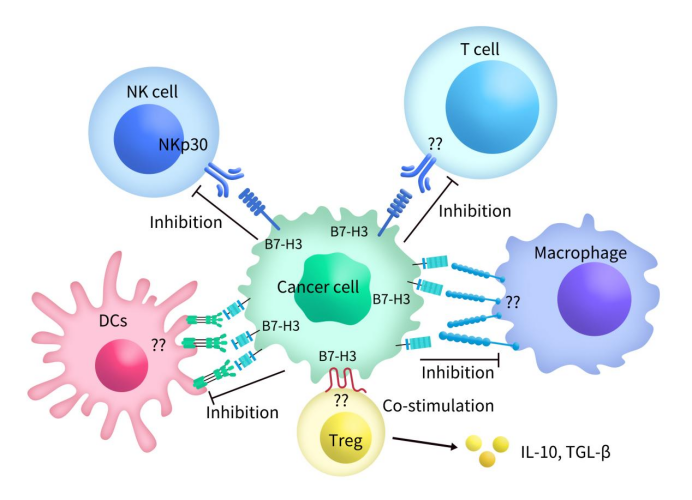
Figure 2. A diagram of the interaction of cancer cell-expressed immune checkpoint B7-H3 with immune cells [11]
In addition to regulating the immune microenvironment, it has been reported that B7-H3 can activate signaling pathways such as ERK, PI3K, and Stat3 in cancer cells, which may lead to enhanced cell proliferation and accelerated tumor growth [12].
3. Advancements in Targeted Therapies for B7-H3
The differential expression of B7-H3 in tumor tissues compared to normal tissues, along with its role in promoting tumorigenesis, has positioned B7-H3 as a promising new immunotherapeutic target. While B7-H3 ADCs are currently receiving considerable attention, research on B7-H3 monoclonal antibodies, bispecific antibodies, and CAR-T cell therapies is also progressing systematically. According to incomplete statistics, there are a total of 109 biopharmaceuticals targeting the B7-H3 target, but only 33 of them are in clinical stages, with ADCs and CAR-T therapies being the primary modalities.
3.1 B7-H3 ADC
Currently, there are a total of 12 B7-H3 ADC drugs in clinical development, with one in Phase III, three in Phase II, three in Phase I, and the remainder in Phase I/II.
Ifinatamab deruxtecan (I-DXd), also known as DS 7300 or DS-7300 or DS-7300A, is a B7H3 ADC developed by Daiichi Sankyo based on its DXd ADC technology platform. It is one of the projects developed in collaboration with Merck & Co. in 2023. In this collaboration, Daiichi Sankyo granted commercialization rights for three DXd ADCs, including I-DXd, outside Japan, for a total amount of $22 billion. I-DXd is composed of a humanized B7-H3 IgG1 antibody and DXd linked by a cleavable linker, MC-GGFG. Its current highest global development status is Phase III clinical trials (NCT06203210). The study aims to evaluate the efficacy and safety of I-DXd compared to other therapies in patients with recurrent small cell lung cancer (SCLC), and it is currently recruiting participants. This drug is also the first B7H3 ADC to enter Phase III trials. Currently, indications under clinical investigation for I-DXd include recurrent SCLC, non-small cell lung cancer, extensive-stage SCLC, advanced solid tumors, castration-resistant prostate cancer, and esophageal squamous cell carcinoma.
Vobramitamab duocarmazine, also known as AEX4089DC1 or MGC 018, is a B7H3 ADC developed by MacroGenics. MGC 018 consists of a B7-H3 IgG1 antibody linked to the cleavable linker Vc-seco-DUBA and the payload Seco-duocarmycin, with a DAR value of 2.7. Its current highest global development status is Phase II clinical trials (NCT06227546 and NCT05551117). The indications include recurrent or refractory extensive-stage small cell lung cancer (ES-SCLC), metastatic castration-resistant prostate cancer, and other solid tumors. The goal of NCT06227546 is to test MGC018 in patients with recurrent or refractory ES-SCLC, with recruitment starting on April 15, 2024. The trial is currently in recruitment phase. NCT06227546 is a Phase II open-label study evaluating MGC 018 in patients with metastatic castration-resistant prostate cancer and other solid tumors, which commenced on June 13, 2023. On April 3, 2024, MacroGenics announced the results of the Phase II clinical trial NCT06227546 at the 2024 ASCO Annual Meeting. As of January 4, 2024, the proportion of patients who discontinued treatment due to adverse reactions in the MGC 018 treatment group (91 cases in the 2.0 mg/kg dose group and 86 cases in the 2.7 mg/kg dose group) was 4.4% and 2.3%, respectively, with no adverse reaction-related deaths reported. Upon the announcement of these results, MacroGenics’ stock price surged by 30% on the same day.
HS-20093 is a B7-H3 ADC developed by Johnson & Johnson Pharmaceutical. It consists of a fully humanized B7-H3 monoclonal antibody covalently linked to a topoisomerase inhibitor (TOPOi) payload via a protease-cleavable linker, with a DAR value of 4. On December 20, 2023, HANSON PHARAM entered into a licensing agreement with GSK, granting GSK exclusive rights outside of China to develop, manufacture, and commercialize HS-20093. While the specific financial terms were not disclosed, Shanghai Johnson Biological will receive an upfront payment of $185 million and may be eligible for up to $1.525 billion in milestone payments for the product. HS-20093’s current highest global development status is Phase II clinical trials (NCT06052423, NCT06112704, and NCT06001255), targeting indications including extensive-stage small cell lung cancer, solid tumors, esophageal cancer, and metastatic castration-resistant prostate cancer. In June 2023, HANSON PHARAM presented the results of a Phase I study of HS-20093 (NCT05276609) at the American Society of Clinical Oncology (ASCO), demonstrating manageable safety, a maximum tolerated dose (MTD) of 12.0 mg/kg, and excellent anti-tumor efficacy in late-stage solid tumor participants who had failed multiple existing standard therapies or were intolerant to standard treatments, particularly demonstrating superior efficacy in small cell lung cancer.
| Drug Category | Drug Name | Development Institution | Highest Development Stage |
| ADC | Ifinatamab deruxtecan | Merck & Daiichi Sankyo | Phase III |
| ADC | Vobramitamab duocarazine | MacroGenics | Phase II |
| ADC | HS-20093 | GSK & HANSON PHARAM | Phase II |
| ADC | YL201 | Medilink therapy | Phase II |
| ADC | 7MW3711 | Mabwell | Phase I/II |
| ADC | DB-1311 | BioNTech & DualityBio | Phase I/II |
| ADC | IBI129 | Innovent Biologics | Phase I/II |
| ADC | IBI3001 | Innovent Biologics | Phase I/II |
| ADC | MHBO88C | Minghui Medicine | Phase I/II |
| ADC | MGC-026 | MacroGenics | Phase I |
| ADC | Mirzotamab clezutoclax | AbbVie, Inc. | Phase I |
| ADC | BAT8009 | BIOTHERA | Phase I |
3.2 B7-H3 CAR-T
Currently, there are a total of 12 B7-H3 CAR-T therapies in clinical development, including 2 autologous CAR-T therapies. Apart from two therapies in Phase I/II clinical trials, the rest are in Phase I clinical trials. Among them, Seattle Children’s Hospital and PersonGen BioTherapeutics (Suzhou) Co., Ltd. each have two B7-H3 CAR-T therapies. Seattle Children’s Hospital’s two CAR-T therapies (B7H3 CAR T Cell and SCRI-CARB7H3) are both in Phase I clinical trials. Unlike SCRI-CARB7H3, B7H3 CAR T Cell is a bispecific CAR-T therapy targeting both CD19 and B7-H3 simultaneously. PersonGen BioTherapeutics’ two CAR-T therapies (TAA6-CAR-T and UTAA06) are also in Phase I clinical trials. Among them, TAA6-CAR-T (also known as TAA06) received special FDA reviews for rare pediatric diseases and orphan drugs in March 2022, targeting neuroblastoma. Additionally, PersonGen BioTherapeutics and Transgene SA reached a collaboration agreement on TAA6-CAR-T therapy in January 2022, although the details of the collaboration have not been disclosed yet.
| Category | Drug Name | Developing Institution | Global Development Stage |
| CAR-T | 4SCAR-276 | Shenzhen Immunogene Therapy Research Institute | Phase I/II |
| CAR-T | Allogenic B7H3 CAR-γδT cell (QH104) | The Fourth Affiliated Hospital of Soochow University | Phase I/II |
| CAR-T | Anti B7 H3 CAR-T-cell therapy (BP-102) | BioPharmGen & Beijing Tian Tan Hospital | Phase I |
| CAR-T | B7 H3 CAR-T-cell therapy (TCB 005) | TC Biopharm Ltd. | Phase I |
| CAR-T | B7-H3-CAR T cells (St. Jude Children’s Research Hospital) | St. Jude Children’s Research Hospital, Inc. | Phase I |
| CAR-T | B7H3 CAR T Cell (Seattle Children’s Hospital) | Seattle Children’s Hospital | Phase I |
| CAR-T | SCRI-CARB7H3 | Seattle Children’s Hospital | Phase I |
| CAR-T | TAA6-CAR-T | PersonGen BioTherapeutics (Suzhou) Co., Ltd. | Phase I |
| CAR-T | UTAA06 | PersonGen BioTherapeutics (Suzhou) Co., Ltd. | Phase I |
| CAR-T | TX-103 | Fuzhou Tuoxin Tiancheng Biotechnology Co., Ltd. | Phase I |
| Autologous CAR-T | B7H3 IL-7Ra CAR T Cell (University of Chulalongkorn) | University of Chulalongkorn | Phase I |
| Autologous CAR-T | CAR.B7-H3T cells (UNC Lineberger Comprehensive Cancer Center) | UNC Lineberger Comprehensive Cancer Center | Phase I |
3.3 B7-H3 Bispecific Antibodies, Monoclonal Antibodies, and Fusion Proteins
Currently, there are a total of 4 bispecific antibody drugs targeting B7-H3 in clinical development, along with 2 monoclonal antibodies and 1 fusion protein. Among them, the most rapidly advancing clinical candidate is Enoblituzumab developed by MacroGenics, Inc., which is currently in Phase II clinical trials, although the trial has been temporarily halted.
Enoblituzumab is an Fc-enhanced monoclonal antibody, a fully-humanized monoclonal antibody containing an Fc domain, designed to enhance its anti-tumor activity by increasing its binding to the activating receptor CD16A and reducing its binding to the inhibitory receptor CD32B. In July 2019, MacroGenics and Zhejiang Teruisi Biopharmaceuticals reached a collaboration agreement worth $150 million, granting Zhejiang Teruisi Biopharmaceuticals exclusive rights for the development and commercialization of Enoblituzumab in mainland China, Hong Kong, Macau, and Taiwan. However, on July 8, 2022, MacroGenics announced the discontinuation of its B7-H3 monoclonal antibody Enoblituzumab in combination with other immune checkpoint inhibitors (PD-1 monoclonal antibodies or PD-1/LAG-3 bispecific antibodies) for the treatment of recurrent and refractory squamous cell carcinoma of the head and neck in a Phase II clinical trial CP-MGA271-06.
| Drug Category | Drug Name | Developing Institution | Global Development Stage |
| Bispecific Antibody | IBI-334 (B7-H3xEGFR) | Innovent Biologics (Suzhou) Co. Ltd. | Phase I/II |
| Bispecific Antibody | TAK-280 (B7-H3xCD3) | Maverick Therapeutics, Inc. | Phase I/II |
| Bispecific Antibody | Orlotamab (B7-H3xCD3) | MacroGenics, Inc. | Phase I |
| Bispecific Antibody | XmAb-808 (B7-H3xCD28) | Xencor, Inc. | Phase I |
| Monoclonal Antibody | Enoblituzumab | MacroGenics, Inc. | Phase II |
| Monoclonal Antibody | IBI-129 | Innovent Biologics (Suzhou) Co. Ltd. | Phase I/II |
| Fusion Protein | IBB0979 (B7-H3xIL-10) | Sunho (China) Biopharmaceutical | Phase I/II |
Additionally, there are currently two antibodies developed by Y-mAbs Therapeutics in clinical trials: the antibody-linked radioisotope 131I-omburtamab and the Monoclonal antibody 8H9 I-124. The former is in the application for marketing stage, while the latter is in Phase II clinical trials.
4. DIMA’s B7-H3 Related Products and Services: Driving the Development of B7-H3 Biopharmaceuticals
DIMA Biotech is a biotechnology company dedicated to preclinical research and development of drug targets. DIMA now offers a full range of products and services related to the B7-H3 target. Our products include active proteins, reference antibodies, and flow cytometry-validated monoclonal antibodies. Services include various species protein antibody customization services, antibody humanization, and affinity maturation services. Additionally, to accelerate the development of B7-H3 biopharmaceuticals, DIMA has prepared a B7-H3 target single B cell seed library, from which lead antibody molecules can be obtained in as little as 28 days. Currently, we have screened over 80 DH17 lead molecules, with nearly 50 of them validated for cross-reactivity with human and monkey proteins. Customers can receive these molecules for functional evaluation and verification the next day. For some molecules, we are also conducting ADC internalization activity and cytotoxicity verification. For specific data, please feel free to contact us.
- B7-H3 Proteins and Antibodies
| Product Types | Cat. No. | Product Name |
| Protein | PME-C100018 | Cynomolgus B7-H3 Protein, His Tag |
| PME-M100008 | Mouse B7-H3 Protein, hFc Tag | |
| PME100012 | Human B7-H3 Protein, mFc-His Tag | |
| PME100755 | Human B7-H3 Protein, mFc Tag | |
| Antibody | DME100053 | Anti-B7-H3 antibody(DM53); Rabbit mAb |
| DME100053B | Biotinylated Anti-B7-H3 antibody(DM53); Rabbit mAb | |
| Reference Antibody | BME100181 | Anti-B7-H3 (TAA06 biosimilar) mAb |
| BME100010 | Anti-B7-H3 (enoblituzumab biosimilar) mAb | |
| BME100010B | Biotinylated Anti-B7-H3 (enoblituzumab biosimilar) mAb | |
| BME100181B | Biotinylated Anti-B7-H3 (TAA06 biosimilar) mAb |
- B7-H3 Lead mAb Molecule Research Progress

References:
[1] Ni, L. & Dong, C. New B7 family checkpoints in human cancers. Mol. Cancer Ther. 16, 1203–1211.
[2] Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001 Mar;2(3):269-74.
[3] Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, Dong C. Characterization of mouse and human B7-H3 genes. J Immunol. 2002 Jun 15;168(12):6294-7.
[4] Kontos F, Michelakos T, Kurokawa T, Sadagopan A, Schwab JH, Ferrone CR, Ferrone S. B7-H3: An Attractive Target for Antibody-based Immunotherapy. Clin Cancer Res. 2021 Mar 1;27(5):1227-1235.
[5] Zhang G, Hou J, Shi J, Yu G, Lu B, Zhang X. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123:538–46.
[6] Sun J, Fu F, Gu W, Yan R, Zhang G, Shen Z, Zhou Y, Wang H, Shen B, Zhang X. Origination of new immunological functions in the costimulatory molecule B7-H3: the role of exon duplication in evolution of the immune system. PLoS One. 2011;6(9):e24751.
[7] Ni L, Dong C. New B7 Family Checkpoints in Human Cancers. Mol Cancer Ther. 2017 Jul;16(7):1203-1211.
[8] Picarda E, Ohaegbulam KC, Zang X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin Cancer Res. 2016 Jul 15;22(14):3425-3431.
[9] Vigdorovich V, Ramagopal UA, Lázár-Molnár E, Sylvestre E, Lee JS, Hofmeyer KA, Zang X, Nathenson SG, Almo SC. Structure and T cell inhibition properties of B7 family member, B7-H3. Structure. 2013 May 7;21(5):707-17.
[10] Han S, Wang Y, Shi X, Zong L, Liu L, Zhang J, Qian Q, Jin J, Ma Y, Cui B, Yang X, Kong B, Zhang Y. Negative roles of B7-H3 and B7-H4 in the microenvironment of cervical cancer. Exp Cell Res. 2018 Oct 1;371(1):222-230.
[11] Getu AA, Tigabu A, Zhou M, Lu J, Fodstad Ø, Tan M. New frontiers in immune checkpoint B7-H3 (CD276) research and drug development. Mol Cancer. 2023 Mar 2;22(1):43.
[12] Ding M, Liao H, Zhou N, Yang Y, Guan S, Chen L. B7-H3-Induced Signaling in Lung Adenocarcinoma Cell Lines with Divergent Epidermal Growth Factor Receptor Mutation Patterns. Biomed Res Int. 2020 Dec 24;2020:8824805.
