Folate receptor alpha (FRα), due to its differential expression in various tumor or cancer tissues compared to normal tissues, is considered an ideal druggable target. In the past, pharmaceutical companies have ventured into developing FRα-targeted therapies, including small molecule conjugates and monoclonal antibody drugs like Morphotek’s humanized monoclonal antibody farletuzumab, and Endocyte’s small molecule conjugate drug vintafolide. Despite their innovative approach, these drugs unfortunately did not succeed in clinical trials. The landscape shifted when antibody-drug conjugates (ADCs) started to revolutionize the pharmaceutical industry, leading to a resurgence of interest in targeting FOLR1 or FRα. Among them, ImmunoGen’s ADC drug, ELAHERE® (mirvetuximab soravtansine-gynx), has shown remarkable progress, receiving FDA accelerated approval in November 2022 for treating FRα-positive platinum-resistant epithelial ovarian cancer, as well as fallopian tube and primary peritoneal cancers in patients who have undergone one to three prior therapies. This success has validated FRα as a viable target for ADCs, prompting pharmaceutical companies to explore additional FRα-targeting strategies, including bispecific antibodies and CAR-T cell therapies. But what is FRα, and how does it influence tumor development? Moreover, what does the competitive landscape look like for biological therapies that target FRα?
1. FRα Structure and Distribution
The folate receptor (FR) family, comprising FRα, FRβ, FRγ, and FRδ, is encoded by the FOLR1, FOLR2, FOLR3, and FOLR4 genes, respectively. All family members, except for FRγ, are single-chain glycoproteins tethered to the cell membrane through glycosylphosphatidylinositol (GPI) anchors. These receptors bind to folate and facilitate its cellular uptake via endocytosis, functioning as key folate transport proteins. As humans are incapable of folate synthesis, dietary intake is essential. Extracellular folate absorption primarily involves three transport proteins: the reduced folate carrier (RFC), encoded by SLC19A1; the proton-coupled folate transporter (PCFT), encoded by SLC46A1; and the FRs. RFC, the predominant transporter, operates as an anion antiporter, leveraging the intracellular organic phosphate gradient to import folate and export organic phosphate. PCFT utilizes the pH gradient, moving folate in concert with protons from the acidic intestinal lumen into the more alkaline interior of intestinal cells. In contrast, FRs, characterized by high affinity but low capacity, selectively mediate folate endocytosis in certain tissues.
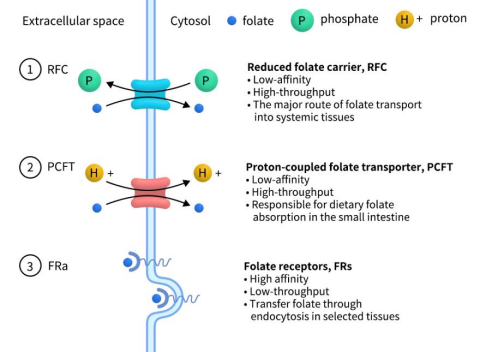
Figure 1. Three types of Folate transporter [1]
The FOLR1 gene, situated on chromosome 11, encodes the FRα protein and is structured with 7 exons and 6 introns. This protein, comprising 257 amino acids, has a molecular weight of approximately 30 kDa. In healthy tissues, FRα expression is confined primarily to the apical surfaces of polarized epithelial cells, such as those in the kidneys, lungs, choroid plexus, reproductive organs, and certain glands [2]. This restricted localization limits its interaction with folate under normal physiological conditions. However, in cancerous tissues, there is a marked overexpression of FRα, particularly in epithelial-derived tumors, which facilitates the increased uptake of folate by the cancer cells.
2. The Role of FRα in Cancer
FRα’s expression is minimal in normal cells, yet it is significantly overexpressed in a variety of solid tumors, including mesothelioma (72-100%), triple-negative breast cancer (35-68%), ovarian cancer (76-89%), and non-small cell lung cancer (14-74%). In contrast, its expression in non-malignant tissues is largely restricted to the epithelial cells lining the apical bronchi. In tumor cells, FRα not only functions as a folate transporter but also acts as a transcription factor, playing a role in cancer cell proliferation and metastasis. As illustrated below, upon binding with folate, FRα initiates a series of intracellular signaling cascades through phosphorylation, activating the ERK and STAT3 signaling pathways, which are crucial regulators of cell growth. Additionally, FRα aids tumor invasion and dissemination by downregulating the intercellular adhesion molecule E-cadherin.
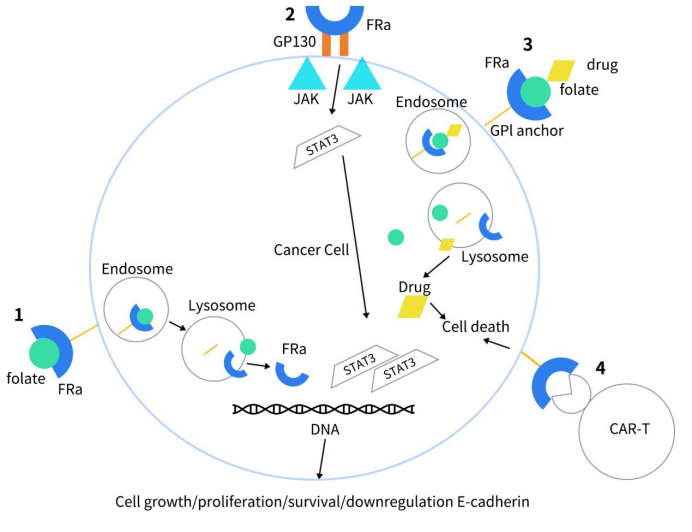
Figure 2. How FRα leads to cell growth [3]
3. Progress in FRα-Targeted Therapies
According to incomplete statistics, there are currently a total of 43 recorded FRα-targeted drugs or therapies worldwide. Among them, 13 are in preclinical stages, 1 has been marketed, and 12 are currently in clinical stages. These therapies encompass various types of drugs, including ADCs, monoclonal antibodies, CAR-T cells, bispecific antibodies, and fusion proteins. Notably, the ADC mirvetuximab soravtansine has marked a significant milestone, being approved for patients with folate receptor alpha-positive ovarian cancer resistant to platinum-based chemotherapy1. The ongoing clinical trials and research into CAR-T cells and vaccines further underscore the commitment to advancing FRα-targeted treatments.
3.1 FRα ADC
The development pipeline for FRα-targeted ADCs is robust, with 21 known ADCs in various stages of development. Of these, 9 are in preclinical research, 7 are advancing through clinical trials, and 1 has achieved market approval. This positions ADCs as the predominant class of emerging drugs aimed at FRα.
Soriatuximab Gedotinib, also known as mirvetuximab soravtansine-gynx or TAK-853, represents a significant advancement in the field of ADCs, specifically targeting the FRα. Developed by ImmunoGen, Inc., this innovative therapy combines an IgG1 subtype anti-FRα humanized monoclonal antibody (M9346A), with the cytotoxic agent DM4—a maytansine derivative—linked via a cleavable linker. As the sole FRα-targeted ADC to receive FDA accelerated approval, it offers a new treatment avenue for adult patients with platinum-resistant epithelial ovarian cancer, fallopian tube cancer, or primary peritoneal cancer who have undergone 1-3 prior systemic therapies and exhibit FRα positivity. The drug’s journey through strategic partnerships began in October 2020 when Hangzhou Zhongmei Huadong Pharmaceuticals secured exclusive rights for its clinical development and commercialization across mainland China, Hong Kong, Macau, and Taiwan, in a deal valued at $305 million. Subsequently, in August 2023, Takeda Pharmaceutical entered into a collaboration and licensing agreement with ImmunoGen, granting Takeda exclusive rights in Japan and providing ImmunoGen with a substantial upfront payment of $340 million. The year culminated with a landmark acquisition in November, as AbbVie purchased ImmunoGen and its flagship oncology therapy, mirvetuximab soravtansine-gynx, for a staggering $10.1 billion, underscoring the drug’s potential and the company’s value in the oncology sector.
Luveltamab Tazevibulin, also known as STRO-002, is an innovative FOLR1/FRα-targeted ADC crafted by Sutro Biopharma. This therapeutic agent is a composite of three distinct elements: an IgG1 subtype anti-FRα monoclonal antibody, the cytotoxic agent SC209 (a semi-synthetic maytansine derivative), and a cleavable linker that releases the drug within the target cells. Leveraging Sutro’s proprietary XpressCF+® technology, the drug is engineered to incorporate non-natural amino acids for precise conjugation, enhancing the ADC’s uniformity and efficacy, reflected in its drug-to-antibody ratio (DAR) of 4. Currently, Luveltamab Tazevibulin is undergoing Phase II/III clinical trials (NCT05870748), which commenced in July 2023. This pivotal study is designed to evaluate the drug’s efficacy and safety against standard investigator’s choice (IC) chemotherapy in patients with FOLR1-expressing ovarian cancer, including those with fallopian tube or primary peritoneal cancer. While awaiting results from this trial, earlier Phase I dose expansion data (NCT03748186) revealed at ASCO 2023 demonstrated a promising overall response rate (ORR) of 37.5% in patients with FRα-positive advanced ovarian cancer.
In a strategic move to expand global access, December 2021 marked the signing of a significant agreement between TASLY Pharmaceutical and Sutro Biopharma. The deal, valued at approximately $385 million, granted TASLY exclusive rights to develop and commercialize Luveltamab Tazevibulin in mainland China, Hong Kong, Macau, and Taiwan. This partnership underscores the potential of Luveltamab Tazevibulin as a frontrunner in the realm of targeted cancer therapies.
AZD-5335 is an ADC drug targeting folate receptor alpha (FRα) developed by AstraZeneca. This therapeutic agent is composed of an FRα-specific antibody linked to AstraZeneca’s novel topoisomerase 1 inhibitor payload, AZ14170132, achieving a DAR of 8. AZD-5335’s primary function is to transport AZ14170132 into FRα-expressing cancer cells, inducing DNA damage and subsequent cell death. The payload also facilitates bystander effects, which are essential for effectively targeting tumors with variable FRα expression levels. Currently, AZD-5335 is undergoing Phase I/IIa clinical trials (NCT05797168), with the objective of assessing its safety, tolerability, and anticancer efficacy, both as a standalone treatment and in combination with other anticancer agents for patients with advanced solid tumors. The trial started in June 2023, and no data has been published yet. However, AstraZeneca presented preclinical data on AZD5335 at AACR 2023. The data showed that at the same or higher doses, AZD5335 exhibited superior preclinical activity compared to a FRα-targeted ADC based on a microtubule inhibitor (MTI) payload in two ovarian cancer patient-derived xenograft models (PDX) with low to moderate FRα expression.
Farletuzumab Ecteribulin, also known as MORAb-202, is a targeted ADC therapy developed by Morab, specifically designed to combat FRα-expressing cancers. This complex drug is composed of the monoclonal antibody farletuzumab, the potent microtubule inhibitor eribulin, and a cleavable linker, achieving a drug-to-antibody ratio (DAR) of 4. This configuration allows for bystander effects, where not only the targeted cancer cells but also neighboring cells can be affected. In a landmark deal in June 2021, Bristol Myers Squibb (BMS) and Morab forged a $3.1 billion agreement, granting BMS the commercial rights to MORAb-202 in the US and Canada. Currently, MORAb-202 is progressing through the clinical trial pipeline, with two significant Phase II trials underway. The first, NCT05613088, is a BMS-led trial assessing MORAb-202’s safety, tolerability, and efficacy against selected chemotherapies in women with platinum-resistant ovarian cancer, including those with primary peritoneal or fallopian tube cancer. The second, NCT05577715, also spearheaded by BMS, aims to determine the safety, tolerability, and objective response rate of MORAb-202 in patients with previously treated metastatic non-small cell lung adenocarcinoma. These trials represent critical steps in establishing MORAb-202 as a viable treatment option for these challenging cancer types.
| Drug Name | Originator Company | Highest Development Stage |
| Mirvetuximab Soravtansine | ImmunoGen, Inc. | Approved for Market |
| Luveltamab Tazevibulin | Sutro Biopharma, Inc. | Phase II/III Clinical |
| AZD-5335 | AstraZeneca PLC | Phase II Clinical |
| Farletuzumab Ecteribulin | Morab/BMS | Phase II Clinical |
| CBP-1008 | Coherentbio | Phase II Clinical |
| Rinatabart Sesutecan | PuFabio Biotech | Phase I/II Clinical |
| AMT-151 | Multitude Therapeutics, Inc. | Phase I Clinical |
| BAT8006 | Bio-Thera Solutions | Phase I Clinical |
Beyond the FRα-targeted ADCs previously discussed, a number of other ADCs are making strides in clinical development. These include Tianyi Pharmaceutical’s CBP-1008, PuFabio Biotech’s Rinatabart Sesutecan (also known as Rina-S or PRO1184), which recently received FDA Fast Track designation, Multitude Therapeutics’ AMT-151, and Baiotai’s BAT8006. Additionally, the ADC landscape is enriched by a promising array of preclinical candidates, such as Iksuda Therapeutics’ IKS-01 and IKS012, Sycivia’s antiFRG ADC, Zymieworks’ ZW191, and Mablink Bioscience’s MBK103. Notably, Mablink Bioscience entered into an acquisition agreement with Lilly in October 2023, a move that underscores the potential of its PSARLink™ technology platform.
3.2 Other Clinical Stage FRα Biologics
In addition to the ADCs targeting FRα, a diverse array of biologics is advancing through clinical trials, including monoclonal antibodies, CAR-T cell therapies and vaccines. Monoclonal antibodies and CAR-T therapies are currently navigating Phase I trials. The research pipeline is further enriched by several bispecific antibodies in preclinical development, such as AFM32 (CD16a/FRα) from Affimed, which leverages innate cell engagement; PT790 (FRα/CD3) and PT796 (FRα/CD47) from Phanes Therapeutics, both designed to harness dual-targeting mechanisms; and TNB-928b from TeneoBio, a T-cell engaging bispecific antibody with a bivalent tumor-selective folate receptor alpha binding arm. These innovative therapies reflect the growing commitment to exploit FRα for therapeutic gains across various platforms.
| Drug Name | Drug Type | Originator Company | Highest Development Stage |
| ITIL-306 | Tumor Infiltrating Lymphocytes | Instil Bio, Inc. | Phase I Clinical |
| MOv18 IgE | Monoclonal Antibody | King’s College London | Phase I Clinical |
| UB-TT170 | CAR-T | Umoja Biopharma, Inc. | Phase I Clinical |
| Folate receptor alpha vaccine | Dendritic Cell Vaccine | Mayo Clinic | Phase II Clinical |
| GALE-302 | Therapeutic Vaccine | Galena Biopharma | Phase II Clinical |
4. DIMA’s FRα-related products and services to empower FRα-targeted therapy development
DIMA Biotechnology LLC is a biotechnology company dedicated to pre-clinical research products and services focusing on potential therapeutic targets. DIMA Biotech now offers a full range of products and services targeting FRα. Our products include active proteins, reference antibodies, and flow cytometry-validated monoclonal antibodies. Our services cover a variety of custom antibody services, antibody humanization, and affinity maturation services. Moreover, to expedite the development of FRα biologic therapies, DIMA Biotech has prepared a FRα target single B cell seed library, with lead antibody molecules available as quickly as 28 days. Additionally, we have screened out 52 CDH17 lead molecules, among which 48 have been validated for cross-reactivity with human and monkey proteins, allowing customers to receive molecules for functional evaluation and validation on the second day. For some molecules, we are also conducting ADC internalization activity and cytotoxicity validation. For specific data, please feel free to inquire.
- FRα/FOLR1 Proteins & Antibodies
| Product types | Cat. No. | Product Name |
| Recombinant Protein | PME100249 | Human FOLR1 Protein, His Tag |
| Recombinant Protein | PME-C100040 | Cynomolgus FOLR1 Protein, His Tag |
| Recombinant Protein | PME-M100114 | Mouse FOLR1 Protein, His Tag |
| WB-Validated Antibody | DMC100585 | Anti-FOLR1 antibody(18A3), IgG1 Chimeric mAb |
| FC-Validated Antibody | DMC100391 | Anti-FOLR1 antibody(DMC391); IgG1 Chimeric mAb |
| Reference Antibody | BME100178 | Anti-FOLR1(mirvetuximab biosimilar) mAb |
| Reference Antibody | BME100163 | Anti-FOLR1(farletuzumab biosimilar) mAb |
| Biotin Labeled Antibody | BME100178B | Biotinylated Anti-FOLR1(mirvetuximab biosimilar) mAb |
| Biotin Labeled Antibody | BME100163B | Biotinylated Anti-FOLR1(farletuzumab biosimilar) mAb |
| Biotin Labeled Antibody | DMC100585B | Biotinylated Anti-FOLR1 antibody(18A3), IgG1 Chimeric mAb |
| Biotin Labeled Antibody | DMC100391B | Biotinylated Anti-FOLR1 antibody(DMC391); IgG1 Chimeric mAb |
- FRα/FOLR1 Lead mAb Molecule Research Progress

Reference:
[1]Mai J, Wu L, Yang L, Sun T, Liu X, Yin R, Jiang Y, Li J, Li Q. Therapeutic strategies targeting folate receptor α for ovarian cancer.
[2]lnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004 Apr 29;56(8):1067-84.
[3]Gonzalez, T.; Muminovic, M.; Nano, O.; Vulfovich, M. Folate Receptor Alpha—A Novel Approach to Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 1046.
