At the 2024 ASCO Annual Meeting, HANGZHOU DAC BIOTECHNOLOGY (abbreviated as DAC Biotech) presented preclinical data on their STEAP1 ADC, DXC008. This ADC targets both STEAP1 and PSMA, showing high affinity for STEAP1 and moderate affinity for PSMA. Preclinical data demonstrated that a single dose of 1 mpk produced durable anti-tumor responses in xenograft models expressing high and moderate levels of STEAP1 and PSMA. However, the renewed interest in the STEAP1 target is not driven by DAC Biotech’s DXC008, but by Amgen’s AMG 509. AMG 509 is a humanized T-cell engager (TCE) molecule targeting STEAP1, which has shown promising results in phase I trials for metastatic castration-resistant prostate cancer (mCRPC), with a monotherapy PSA50 of 49% and an objective response rate (ORR) of 24%. Although toxicity was slightly elevated, the overall safety profile was considered acceptable. What makes STEAP1 an intriguing target? And how is the landscape of clinical therapeutics evolving in this area?
1. Structure and Function of STEAP1
Six-transmembrane epithelial antigen of the prostate 1 (STEAP1), also known as PRSS2 or STEA1, belongs to the six-transmembrane epithelial antigen of the prostate (STEAP) family, which also includes STEAP1B, STEAP2, STEAP3, and STEAP4. STEAP1B is a truncated homolog of STEAP1, a tetraspanning membrane protein. STEAP1-4 are metalloproteins that play crucial roles in iron and copper homeostasis [1]. The STEAP1 gene is located on chromosome 7q21.13 and consists of 5 exons and 4 introns, spanning 10.4 kb. Transcription of the STEAP1 gene produces mRNA transcripts at 1.4 and 4.0 kb, with only the 1.4 kb mRNA encoding the STEAP1 protein. The STEAP1 protein comprises 339 amino acids with a molecular weight of 39.72 kDa, representing the first reported STEAP protein. STEAP1 protein features six transmembrane domains, with both N- and C-termini located on the cytoplasmic side [2]. The C-terminus of STEAP1, like STEAP2-4, contains a ferrireductase (FRE) domain with bound heme b. However, unlike STEAP2-4, the N-terminus of STEAP1 lacks an NADPH-binding FNO domain, thus STEAP1 cannot independently exhibit Fe3+ or Cu2+ reductase activity [3].
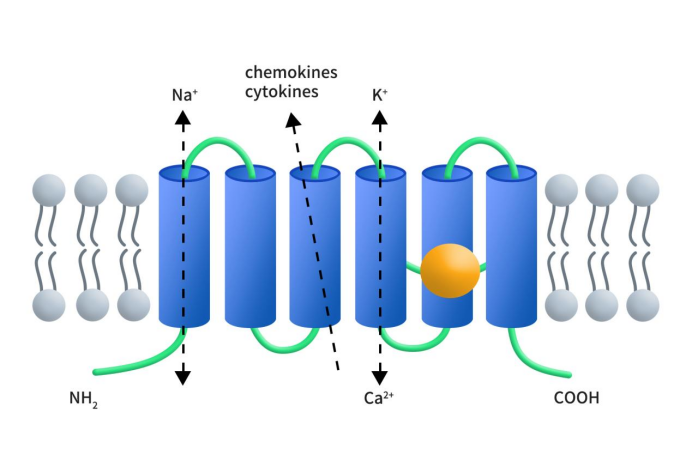
Figure 1. The structure of STEAP1 [2]
The STEAP family proteins share structural similarities and are localized on the cell membrane and within the cytoplasm. STEAP1-4 proteins, found on the plasma membrane surface, each feature six potential transmembrane regions and hydrophilic amino and carboxyl termini intracellularly, suggesting roles as channels or transporters. Both STEAP1 and STEAP2 proteins on the membrane contain at least one heme group, which may be involved in iron and copper absorption [5]. As mentioned earlier, STEAP1 lacks an NADPH-binding FNO domain and thus cannot independently reduce metals. However, research indicates that STEAP1 can exhibit cellular ferrireductase activity through fusion with STEAP4’s intracellular NADPH-binding domain [4]. The secondary structure of STEAP1 is related to its location at the junctions of prostatic secretory epithelial cells, suggesting its role in intercellular communication, modulation of small molecules and ions (such as Na+, Ca2+, and K+), and the release of soluble cytokines and chemokines. Recent studies have also shown that STEAP1 can form both homotrimers and heterotrimers with STEAP2, which may influence its function and localization on the plasma membrane. This interaction highlights the complexity of STEAP1’s role in cellular processes and its potential as a therapeutic target in cancer treatment.
2. STEAP1 and Cancer
Compared to normal prostate tissue, STEAP1 is overexpressed in malignant prostate tissue. Current research on STEAP1 primarily focuses on prostate cancer, where its overexpression promotes tumor growth, while its knockdown inhibits prostate tumor growth. Beyond prostate cancer, elevated STEAP1 expression has been observed in other tumor tissues including renal cell carcinoma, bladder cancer, Ewing sarcoma, breast cancer, colorectal cancer (CRC), gastric cancer, ovarian cancer, and lung cancer. Although the specific mechanisms of STEAP1 in cancer require further investigation, some studies have begun to uncover certain mechanisms. In prostate cancer, STEAP1 acts as a cell surface membrane protein essential for intercellular communication between tumor cells and adjacent stromal cells, thereby promoting tumor growth [6]. In androgen-dependent prostate cancer, knocking down STEAP1 can inhibit cell growth and induce apoptosis in LNCaP prostate cancer cells in a dihydrotestosterone (DHT)-independent manner [7]. In lung adenocarcinoma, knocking down STEAP1 significantly inhibits proliferation and migration of lung adenocarcinoma cells. STEAP1 regulates epithelial-mesenchymal transition (EMT) through the JAK2/STAT3 signaling pathway [8]. In colorectal cancer (CRC), high levels of STEAP1 transcription reduce ROS production, thereby preventing CRC cells from undergoing apoptosis via the NRF2 pathway [9]. In hepatocellular carcinoma, knocking down STEAP1 inhibits c-Myc expression, causing cancer cells to arrest in the G1 phase and suppressing cell proliferation [10].
Recent studies have highlighted STEAP1’s potential as a therapeutic target due to its tumor-specific expression and membrane-bound localization. Clinical trials are exploring antibody-drug conjugates (ADCs) and chimeric antigen receptor (CAR) T-cell therapies targeting STEAP1, showing promising results in treating metastatic castration-resistant prostate cancer.
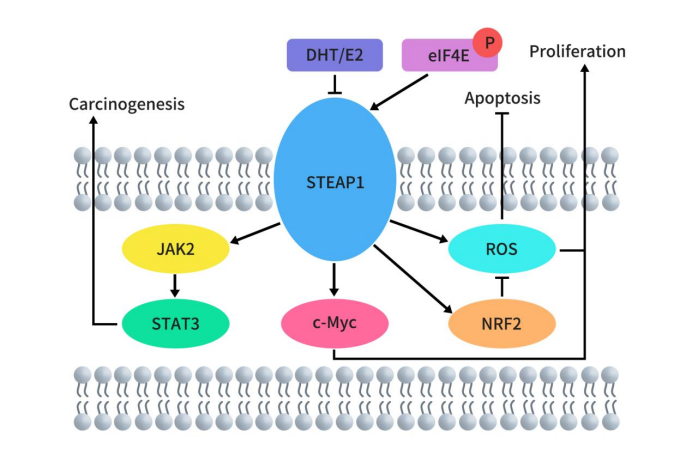
Figure 2. Molecular mechanisms of STEAP1 in cancer [5]
3. Clinical Advances in STEAP1 Targeted Therapy
Currently, there are several STEAP1-targeted therapies in clinical development, including antibodies, CAR-T cells, and antibody-drug conjugates (ADCs). Among these, the most advanced are in Phase II trials.
- 89Zr-DFO-MSTP2109A
89Zr-DFO-MSTP2109A is a radiolabeled antibody targeting STEAP1, jointly developed by Genentech, Inc. and Memorial Sloan Kettering Cancer Center. Clinical trials Phase 1/2 (NCT01774071) for 89Zr-DFO-MSTP2109A have been completed. The results indicated that 89Zr-DFO-MSTP2109A was well tolerated by patients, with no significant toxicity observed. The antibody showed excellent uptake in bone and soft tissue metastases in metastatic castration-resistant prostate cancer (mCRPC) patients, with a median SUV max of 20.6 in bone and 16.8 in soft tissue. Sixteen of 17 lesions biopsied were positive on 89Zr-DFO-MSTP2109A imaging, and all sites were histologically positive. These findings suggest that 89Zr-DFO-MSTP2109A has potential as a diagnostic agent for detecting STEAP1-expressing tumors and warrants further exploration as a companion diagnostic in patients undergoing STEAP1-directed therapy.
- STEAP1 CAR-T Cell Therapy
STEAP1 CAR-T Cell Therapy is a treatment developed jointly by PromiCell Therapeutics Inc. and Fred Hutchinson Cancer Research Center. STEAP1 CAR-T cells are engineered to target prostate tumor cells more effectively by modifying them to recognize the STEAP1 antigen. Currently, a Phase I/II clinical trial (NCT06236139) is underway to evaluate the safety and efficacy of combining STEAP1 CAR-T cell therapy with enzalutamide in patients with metastatic castration-resistant prostate cancer (mCRPC). This clinical trial was first announced on February 1, 2024, and recruitment has not yet commenced.
- AMG-509
AMG-509, also known as xaluritamig, is a bispecific T cell engager (BiTE) developed collaboratively by Amgen and BeiGene. It simultaneously targets STEAP1 and CD3 receptors on T cells, binding to both the STEAP1 antigen in humans and non-human primates and the CD3 receptor on T cell surfaces. Structurally, Amgen utilized Xencor’s Xmab 2+1 asymmetric technology and extended the half-life of this protein. As depicted in the figure below, AMG-509 consists of two identical humanized anti-STEAP1 Fab domains and one anti-CD3 scFv domain, with the anti-CD3 scFv domain linked to one end of the STEAP1 Fab domain. Preliminary preclinical data released on October 20, 2023, for treating prostate cancer demonstrated potent T cell-dependent cytotoxicity against prostate cancer cell lines in vitro and promoted tumor regression in xenograft and syngeneic mouse models of prostate cancer [11]. Currently, AMG-509 is in Phase I clinical trials. Interim results from these trials have shown a positive benefit/risk profile, with robust anti-tumor activity in heavily pretreated patients with metastatic castration-resistant prostate cancer (mCRPC). The most common treatment-related adverse events were cytokine release syndrome (CRS), fatigue, and myalgia, with CRS occurring primarily during the first cycle and improving with premedication and step dosing. Encouraging prostate-specific antigen (PSA) and RECIST responses were observed, supporting further development of AMG-509.
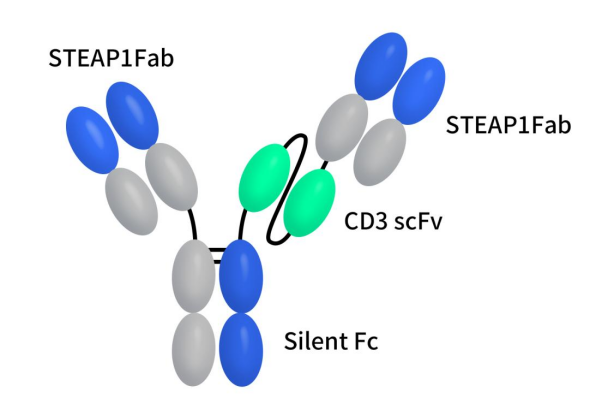
Figure 3. The structure of AMG-509
- ABBV-969
ABBV-969 is a dual-targeted antibody-drug conjugate (ADC) developed by AbbVie. It targets both PSMA and STEAP1 using a topoisomerase inhibitor as its payload. Currently, ABBV-969 is initiating a Phase I clinical trial (NCT06318273) for patients with metastatic castration-resistant prostate cancer (mCRPC). This clinical trial is expected to commence in March 2024, with a planned enrollment of 120 participants. The study aims to assess the safety, pharmacokinetics, and preliminary efficacy of ABBV-969 as a monotherapy. Participants will receive escalating doses of ABBV-969 to determine the recommended Phase II dose. The trial will also evaluate the drug’s ability to target and eliminate cancer cells expressing PSMA and STEAP1, potentially offering a new therapeutic option for patients with mCRPC.
4. DIMA’s STEAP1-Related Products Facilitating Biopharmaceutical Development
DIMA Biotech is a biotechnology company specializing in preclinical development products and services focused on drug targets. Over the next 3-5 years, the company plans to finalize the development of lead antibody molecules for all druggable targets. This strategic initiative aims to assist pharmaceutical companies in overcoming the challenges associated with establishing monoclonal antibody platforms and conducting lead antibody molecule screening. By streamlining these processes, DIMA Biotech aims to allow more time for pharmaceutical companies to concentrate on the biological mechanisms and druggability research of their drug targets, thereby accelerating progress in clinical pipelines.
Currently, DIMA Biotech offers a comprehensive range of products and services targeting STEAP1. This includes bioactive proteins and specific antibodies. Of special significance is the successful production of the full-length human STEAP1 protein using synthetic nanodisc technology. This protein is expressed in HEK293 cells and purified using advanced nanodisc technology. DIMA Biotech has verified the protein’s quality through ELISA and SDS-PAGE methods, ensuring its suitability for research and development purposes.
| Product Type | Cat.No. | Product Name |
| Full-length Protein | FLP100070 | Human STEAP1 full-length protein-synthetic nanodisc |
| ECD Protein | PME101530 | Human STEAP1 Protein, hFc Tag |
| Reference Antibody | BME100208 | Anti-STEAP1(xaluritamig without CD3 biosimilar) mAb |
| BME100188 | Anti-STEAP1(Vandortuzumab biosimilar) mAb | |
| Biotin-labeled Antibody | BME100208B | Biotinylated Anti-STEAP1(xaluritamig without CD3 biosimilar) mAb |
| BME100188B | Biotinylated Anti-STEAP1(Vandortuzumab biosimilar) mAb |
References:
[1]Ohgami R.S., Campagna D.R., McDonald A., et al. The Steap proteins are metalloreductases. Blood. 2006;108:1388–1394.
[2]Barroca-Ferreira J, Pais JP, Santos MM, et al. Targeting STEAP1 Protein in Human Cancer: Current Trends and Future Challenges. Curr Cancer Drug Targets. 2018;18(3):222-230.
[3]Xu M, Evans L, Bizzaro CL, et al. STEAP1-4 (Six-Transmembrane Epithelial Antigen of the Prostate 1-4) and Their Clinical Implications for Prostate Cancer. Cancers (Basel). 2022 Aug 20;14(16):4034.
[4]Oosterheert W, Gros P. Cryo-electron microscopy structure and potential enzymatic function of human six-transmembrane epithelial antigen of the prostate 1 (STEAP1). J Biol Chem. 2020 Jul 10;295(28):9502-9512.
[5]Chen WJ, Wu HT, Li CL, et al. Regulatory Roles of Six-Transmembrane Epithelial Antigen of the Prostate Family Members in the Occurrence and Development of Malignant Tumors. Front Cell Dev Biol. 2021 Oct 29;9:752426.
[6]Yamamoto T, Tamura Y, Kobayashi J, et al. Six-transmembrane epithelial antigen of the prostate-1 plays a role for in vivo tumor growth via intercellular communication. Exp Cell Res. 2013 Oct 15;319(17):2617-26.
[7]Gomes IM, Rocha SM, Gaspar C, et al. Knockdown of STEAP1 inhibits cell growth and induces apoptosis in LNCaP prostate cancer cells counteracting the effect of androgens. Med Oncol. 2018 Feb 20;35(3):40.
[8]Huo SF, Shang WL, Yu M, et al. STEAP1 facilitates metastasis and epithelial-mesenchymal transition of lung adenocarcinoma via the JAK2/STAT3 signaling pathway. Biosci Rep. 2020 Jun 26;40(6):BSR20193169.
[9]Nakamura H, Takada K, Arihara Y, et al. Six-transmembrane epithelial antigen of the prostate 1 protects against increased oxidative stress via a nuclear erythroid 2-related factor pathway in colorectal cancer. Cancer Gene Ther. 2019 Sep;26(9-10):313-322.
[10]Iijima K, Nakamura H, Takada K, et al. Six-transmembrane epithelial antigen of the prostate 1 accelerates cell proliferation by targeting c-Myc in liver cancer cells. Oncol Lett. 2021 Jul;22(1):546. doi: 10.3892/ol.2021.12807.
Nolan-Stevaux O, Li C, Liang L, et al. AMG 509 (Xaluritamig), an Anti-STEAP1 XmAb 2+1 T-cell Redirecting Immune Therapy with Avidity-Dependent Activity against Prostate Cancer. Cancer Discov. 2024 Jan 12;14(1):90-103.
