On September 25, 2024, I-Mab announced a strategic collaboration with Sanofi to develop, produce, and commercialize uliledlimab in Greater China. This partnership, valued at approximately €213 million (around 1.7 billion RMB), aims to leverage Sanofi’s expertise in oncology to accelerate the development and commercialization of uliledlimab, which is an innovative CD73 monoclonal antibody independently developed by I-Mab, designed to enhance the body’s immune response to cancer cells by modulating the tumor microenvironment. Today, we will discuss the biological background of CD73 and the progress of research on targeted therapies.
1. Structure of CD73
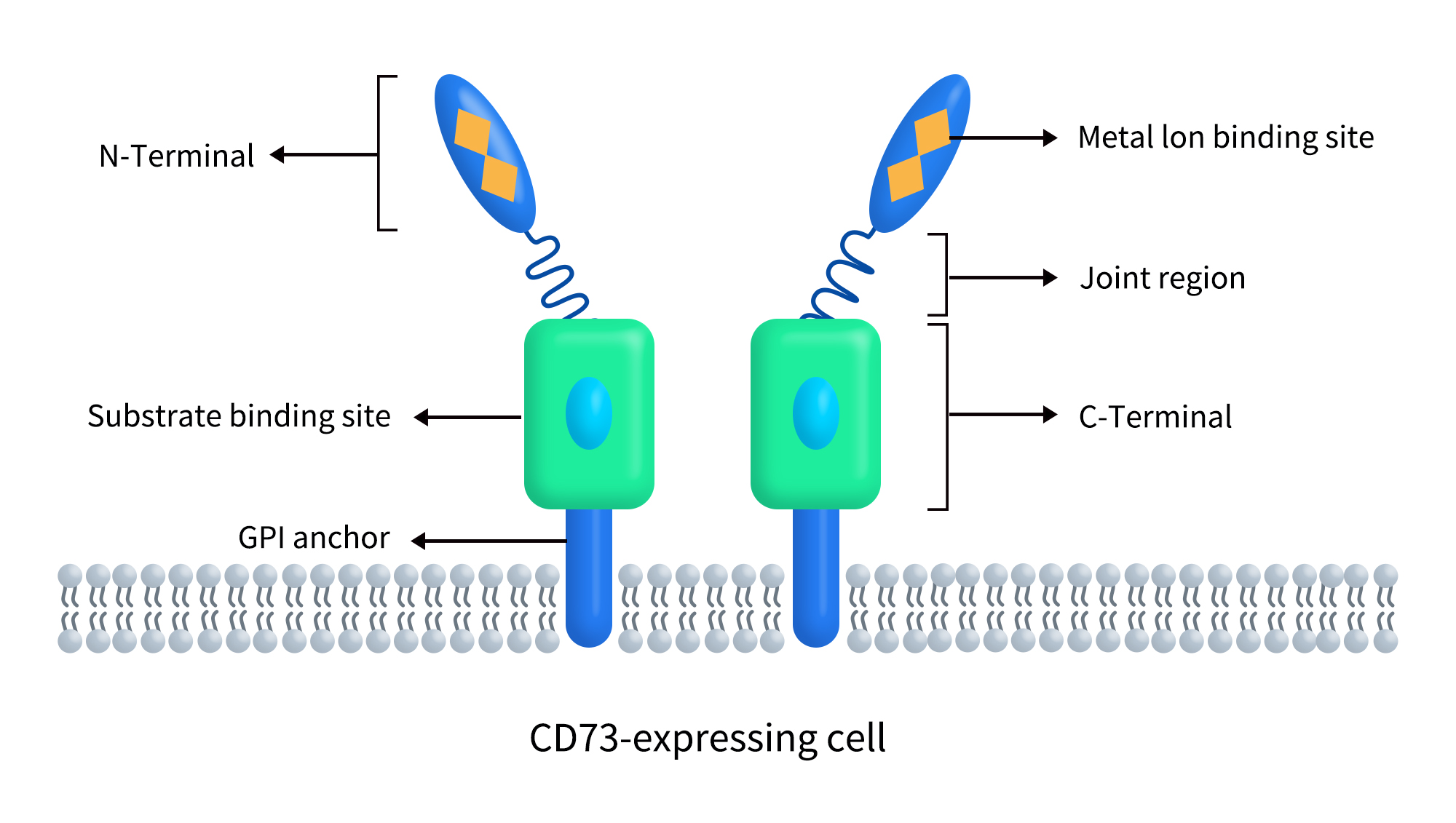
CD73, also known as ecto-5’-nucleotidase or 5’-nucleotidase (5’-NT), is a glycosylphosphatidylinositol (GPI)-anchored cell surface protein encoded by the NT5E gene. The human NT5E gene is located on chromosome 6q14.3 and encodes a CD73 protein of 574 amino acids. As shown in Figure 1, CD73 is a dimeric ecto-5’-nucleotidase (5’-NT) expressed on the extracellular side of the plasma membrane. Each subunit comprises three structural domains, with the N-terminus containing two metal ion binding sites and the C-terminus featuring a substrate binding site. An α-helix forms a flexible linker region, and CD73 is attached to the cell membrane via a GPI anchor [1].
Figure 1. The structure of CD74 [1]
2. Distribution and Function of CD73
CD73 is present on the surface of various cell types, including endothelial cells, lymphocyte subtypes, stromal cells, and certain types of tumor cells. It plays a critical role in the conversion of extracellular 5′-adenosine monophosphate (5′-AMP) into adenosine, making it an important component of the purinergic signaling pathway. This process is facilitated by the action of CD39, which catalyzes the hydrolysis of ATP and ADP into AMP.
Extracellular ATP is rapidly metabolized by several enzymes collectively known as ecto-nucleotide triphosphate diphosphohydrolases (ENTPD) and members of the ecto-nucleotide pyrophosphatase/phosphodiesterase (ENPP) protein family. In addition to CD39, ENPP1 can also catalyze the conversion of ATP to AMP. Subsequently, CD73 hydrolyzes AMP into adenosine.
Adenosine can activate four types of G protein-coupled adenosine receptors (AR), including A1R (ADORA1), A2AR (ADORA2A), A3R (ADORA3), and A2BR (ADORA2B). These receptors have varying affinities for adenosine, with the order of affinity from highest to lowest being: A1R, A2AR, A3R, and A2BR, where the first three receptors have significantly higher affinities than A2BR. Activation of these receptors can trigger multiple signaling pathways that regulate oxygen supply and demand, inflammation, and other physiological activities, depending on the cell type and receptor expression patterns [2]. Furthermore, adenosine can also be taken up via equilibrative nucleoside transporters (ENT) and re-phosphorylated to AMP within the cell [3]. As a primary source of extracellular adenosine, CD73 is a key regulator of cellular homeostasis, stress responses, injury, and disease mechanisms in various tissues [4].
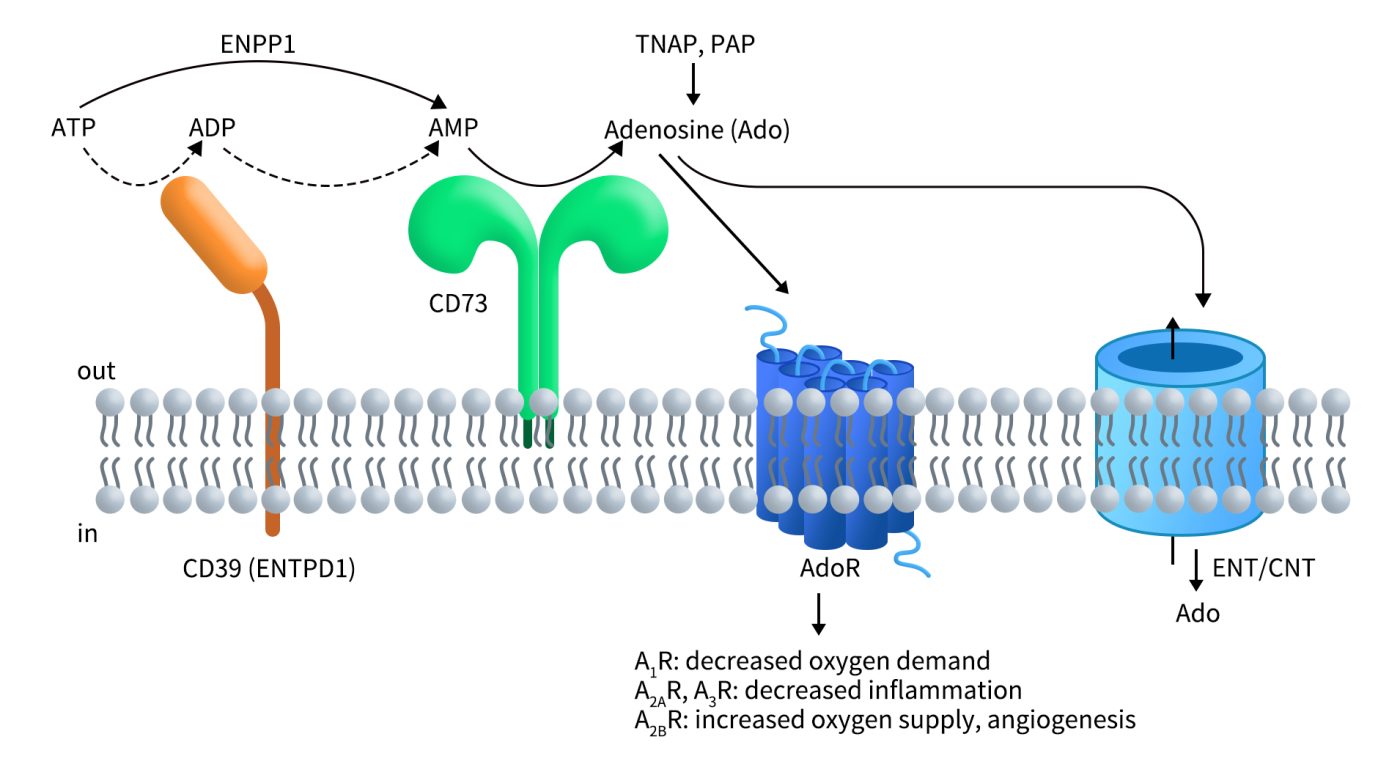
Figure 2. CD73 is an essential component of purinergic signaling [5]
3. CD73 and Tumors
In recent years, the purinergic signaling pathway has emerged as a significant player in cancer progression, with extracellular ATP, ADP, and adenosine serving as key signaling molecules [6]. Adenosine, an immunosuppressive metabolite, is a component of the tumor microenvironment (TME). As the primary enzyme catalyzing adenosine production, CD73 is crucial for suppressing adequate anti-tumor immune responses by generating adenosine, while also promoting cancer cell proliferation, tumor growth, angiogenesis, and metastasis.
As illustrated in Figure 3, ATP released from damaged or dying cells in the TME acts as a danger signal for immune cells, primarily signaling through P2X and P2Y receptors, which are differentially expressed across various cell types to enhance anti-tumor immune responses. ATP is subsequently degraded to adenosine through the cooperative action of CD39 and CD73. Adenosine inhibits immune cells via A2A and A2B receptor signaling or promotes tumor growth by directly enhancing cancer cell proliferation, angiogenesis, epithelial-to-mesenchymal transition (EMT), and metastasis.
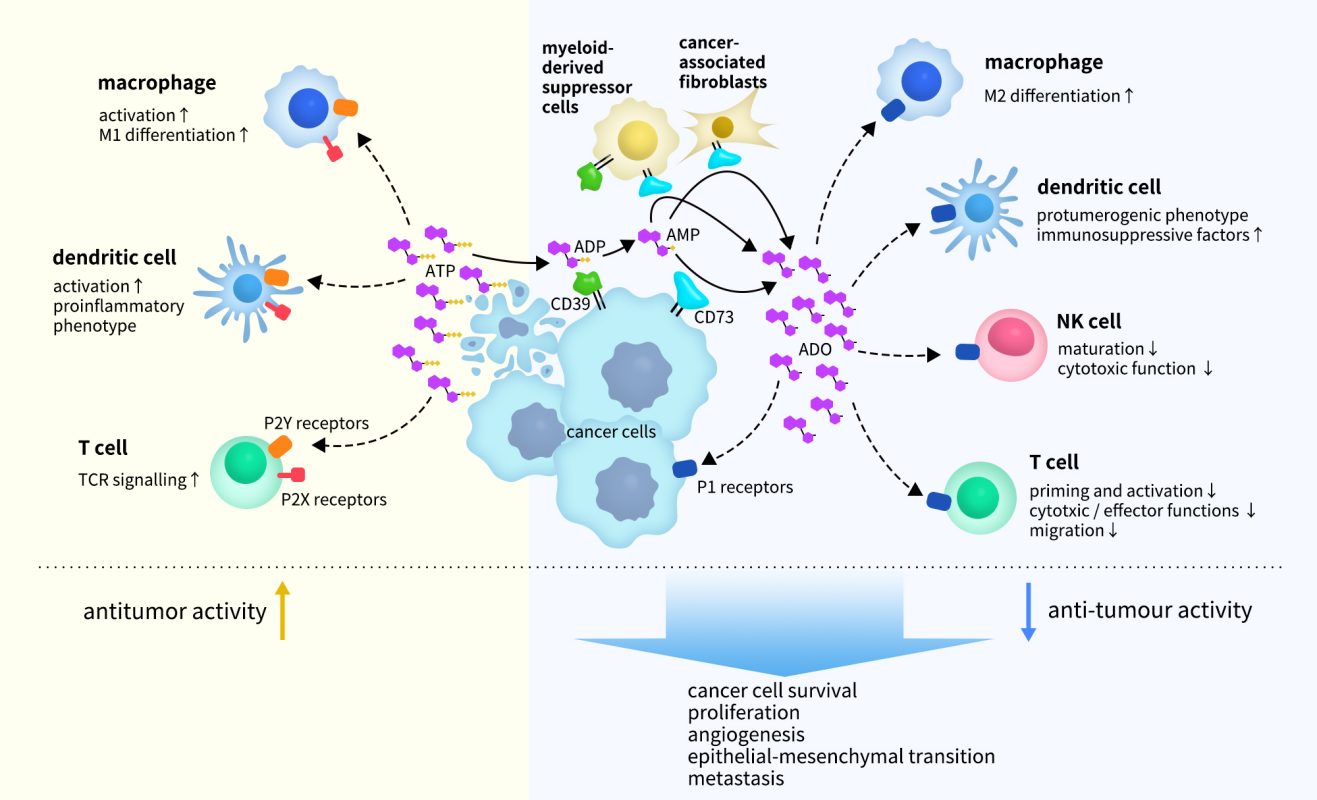
Figure 3. ATP and adenosine signaling in the tumor microenvironment (TME) [7]
CD73 is overexpressed in various cancer cell lines and patient biopsies, though the levels of expression vary among different cancer types. It is found to be highly expressed in glioblastoma, thyroid cancer, sarcoma, pancreatic cancer, gastric adenocarcinoma, colorectal cancer, renal cell carcinoma, esophageal cancer, thymoma, rectal adenocarcinoma, lung adenocarcinoma, non-small cell lung cancer, and acute myeloid leukemia. In contrast, lower levels of CD73 expression have been observed in urogenital cancers, as well as in melanoma, breast cancer, and cholangiocarcinoma [7].
4. Clinical Research Progress of CD73-targeted Drugs
Increasing evidence indicates that CD73 is a key regulatory molecule in cancer cell proliferation, migration, and invasion (in vitro), as well as tumor angiogenesis and immune evasion (in vivo). Based on these roles, CD73 has emerged as an attractive therapeutic target. A preliminary count shows that there are a total of 89 drugs targeting CD73 globally, including 34 in preclinical development, 26 in clinical stages, and 5 in the clinical application approval stage.
Although no CD73-targeted drugs are currently on the market, the diversity of drug types—including monoclonal antibodies, antibody-drug conjugates (ADCs), bispecific antibodies, small molecule inhibitors, and fusion proteins—demonstrates the significant potential of the CD73 target. The most numerous drug type in development is monoclonal antibodies, and here we highlight the research progress of CD73-targeted drugs that are in clinical trials or at the clinical application stage.
4.1 CD73 Monoclonal Antibodies
Currently, there are 18 CD73-targeted monoclonal antibody drugs in clinical development globally, including one antibody (S-095024) whose specific type is not specified. Among these 18 clinical-stage drugs, 9 are in Phase 1, 2 are in Phase 2, and only 1 is in Phase 3. The leading companies in this space include AstraZeneca, Halozyme Therapeutics, and Bristol Myers Squibb (BMS) internationally, while in China, prominent players include I-Mab Biopharma, Innovent, and Harbour BioMed.
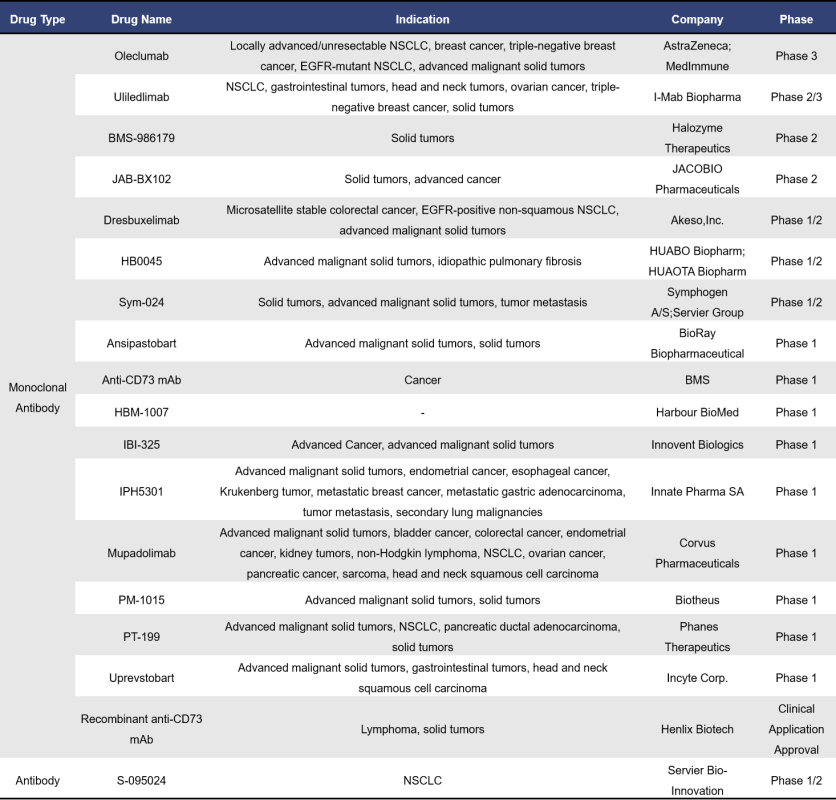
Oleclumab is a fully humanized IgG1λ CD73 monoclonal antibody (mAb) developed by AstraZeneca, and it is the fastest progressing CD73 mAb in global clinical development. The heavy chain constant region of Oleclumab has undergone triple mutations, resulting in reduced IgG effector functions, which induces the internalization of CD73 to suppress its activity. In a mouse model of colorectal cancer, Oleclumab alleviated AMP-induced T cell proliferation inhibition, significantly suppressed tumor growth, increased the percentage of CD8+ T cells and myeloid cells within tumor infiltration, and enhanced the potential for antigen presentation. Currently, Oleclumab is being developed for the treatment of various solid tumors, including non-small cell lung cancer (NSCLC) and pancreatic cancer, with the highest stage of development being Phase III.
Uliledlimab is a highly differentiated humanized IgG1κ CD73 monoclonal antibody (mAb) independently developed by I-Mab Biopharma. It is currently the second most advanced CD73 mAb in global clinical development. This antibody has good tolerability with no dose-limiting toxicities and is capable of saturating circulating and cell-bound CD73, enhancing the immune response against cancer cells by modulating the tumor microenvironment. At the 2023 ASCO Annual Meeting, I-Mab presented data from its Phase 1b/2 clinical studies conducted in the United States and China. The results showed that the combination of Uliledlimab with toripalimab demonstrated good safety and significant efficacy in patients with CD73-high expressing non-small cell lung cancer (NSCLC). The highest stage of development globally is currently Phase 2/3.
S-095024 is a CD73 antibody drug with unspecified antibody type, developed by Servier Bio-Innovation. This fully humanized antibody has reduced effector functions and can bind to human and crab-eating macaque CD73 with sub-nanomolar affinity. Early clinical data presented at the 2024 ASCO Annual Meeting revealed the potential of S-095024 to block adenosine-mediated tumor escape, supporting further clinical research.
4.2 CD73 Bispecific Antibodies
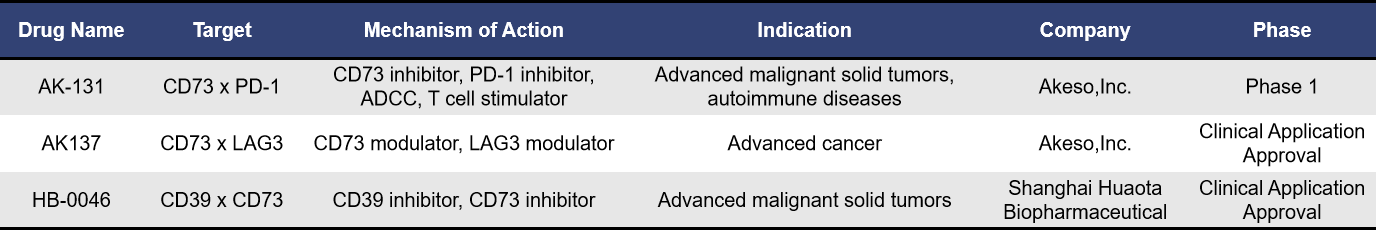
A representative bispecific antibody targeting CD73 is AK131 from Akeso,Inc., which is also the only CD73 bispecific antibody currently in clinical development globally. AK131 is designed to target both PD-1 and CD73, derived from AK105 (PD-1 monoclonal antibody) and AK119 (CD73 monoclonal antibody). AK119 not only inhibits CD73 activity and adenosine production but also stimulates B cell activation. AK131 effectively and completely suppresses the enzymatic activity of CD73, blocking PD-1-mediated immune cell inhibition.
Data presented at the 2022 AACR Annual Meeting demonstrated that AK131 exhibits strong in vitro and in vivo activity. In addition to effectively blocking the PD-1/PD-L1 interaction, AK131 also promotes the activation of T cells and B cells and induces the internalization of CD73.
4.3 Small Molecule Inhibitors Targeting CD73
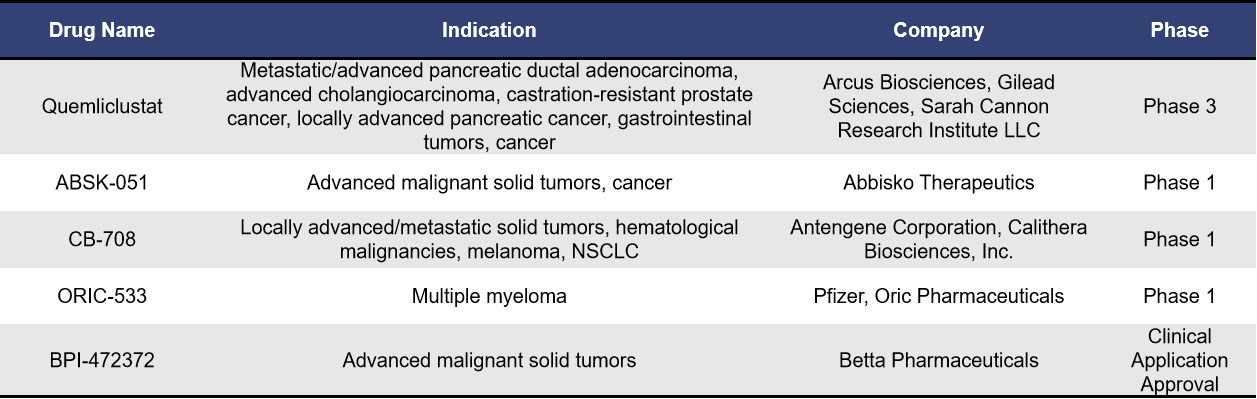
Currently, there are five small molecule inhibitors targeting CD73 in clinical development, with Quemliclustat being the fastest progressing globally. Developed by Arcus Biosciences, Quemliclustat is a small molecule inhibitor that effectively blocks adenosine production in the tumor microenvironment (TME), thereby inhibiting AMP and extracellular adenosine-mediated tumor immune suppression. On September 23, 2024, it entered Phase III clinical trials (PRISM-1/NCT06608927), becoming the first small molecule CD73 inhibitor to reach this stage.
The NCT06608927 trial aims to evaluate the efficacy and safety of Quemliclustat in combination with chemotherapy (albumin-bound paclitaxel + gemcitabine) compared to placebo plus chemotherapy in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (PDAC).
4.4 CD73 ADC
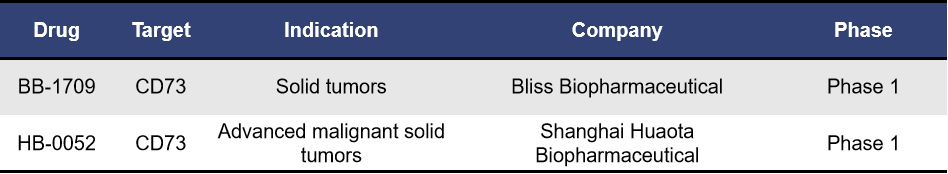
Currently, there are two CD73 ADCs in clinical development: BB-1709 from BlissBio and HB-0052 from Huaota Biopharma, both of which are in Phase 1 trials. Research has shown that compared to unconjugated CD73 antibodies, CD73 ADCs can significantly induce the accumulation of pro-inflammatory macrophages and activated dendritic cells (DCs) within tumors. Additionally, CD73 ADCs possess protective effects on T cells and stimulate DCs, providing a dual advantage in targeting CD73 high-expressing tumors and enhancing tumor immune responses. However, specific effects still require further clinical validation.
5. DIMA’s CD73-Related Products and Services Supporting CD73 Biotherapy Development
DIMA Biotechnology is a biotechnology company focused on providing preclinical research products and services for druggable targets. DIMA now offers a comprehensive range of products and services targeting CD73. The products include active proteins, reference antibodies, and flow validation monoclonal antibodies. Services encompass custom antibody development for various species, antibody humanization, and affinity maturation.
To accelerate the development of CD73 biotherapies, Dima has also established a CD73 single B cell seed library, allowing for lead antibody molecules to be obtained in as little as 28 days. Additionally, we have screened multiple CD73 lead molecules, which clients can access for functional evaluation as soon as the next day.
- CD73 protein&antibody&CDX slide
- Progress on CD73 Lead mAb Molecules

Reference:
[1] Zandieh, K., Millani, S., Mohammadi, J., et al. Therapeutic Value of CD73 as a Biomarker in Human Cancer. Archives of Advances in Biosciences, 2019, 10(3), 45–54.
[2] Borea PA, Gessi S, Merighi S, et al. Pharmacology of Adenosine Receptors: The State of the Art. Physiol Rev. 2018 Jul 1;98(3):1591-1625.
[3] Pastor-Anglada M, Pérez-Torras S. Who Is Who in Adenosine Transport. Front Pharmacol. 2018 Jun 14;9:627.
[4] Minor M, Alcedo KP, Battaglia RA, et al. Cell type- and tissue-specific functions of ecto-5′-nucleotidase (CD73). Am J Physiol Cell Physiol. 2019 Dec 1;317(6):C1079-C1092.
[5] Alcedo, Karel P. et al. The elegant complexity of mammalian ecto-5’-nucleotidase (CD73). Trends in Cell Biology, 31 (10), 829 – 842.
[6] Burnstock G. and Di Virgilio F., Purinergic signalling and cancer, Purinergic Signalling. (2013) 9, no. 4, 491–324.
[7] Bach, N., Winzer, R., Tolosa, E., et al. The Clinical Significance of CD73 in Cancer. International journal of molecular sciences, 2023, 24(14), 11759.
[8] Jin R, Liu L, Xing Y, et al. Dual Mechanisms of Novel CD73-Targeted Antibody and Antibody-Drug Conjugate in Inhibiting Lung Tumor Growth and Promoting Antitumor Immune-Effector Function. Mol Cancer Ther. 2020 Nov;19(11):2340-2352.