Project#: LA100002
Target:BCMA
Synonyms:BCM; BCMA; CD269; TNFRSF13A; TNFRSF17
Uniprot ID:Q02223
Project stage:We have identified and prevalidated over 50 lead monoclonal antibody (mAb) sequences, including original rabbit monoclonal antibodies, humanized antibodies, and CAR-molecules developed based on humanized sequences. These antibodies have undergone comprehensive validation, including in vitro killing assays, to assess their binding affinity and functional efficacy. Based on these results, several of these lead antibodies demonstrate strong potential for further development into Antibody-Drug Conjugates (ADCs) or CAR-T therapies.
Indications: Multiple Myeloma; Autoimmune Diseases
Licensing Opportunities: Sequence licensing for different applications.
LA100002-BCMA Lead Antibodies
Project Overview
Antibody Sequence Licensing Request Form
Background
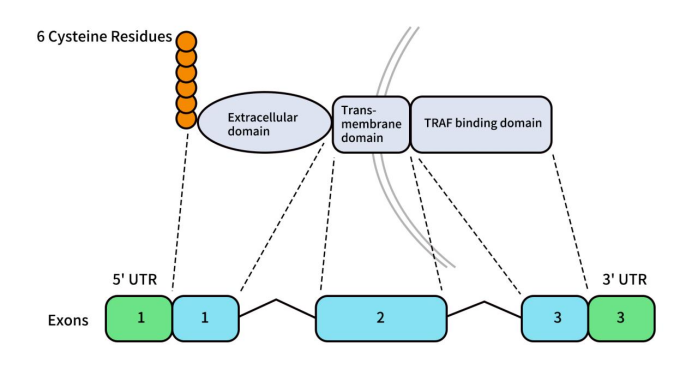
Human BCMA, also known as TNFRSF17, CD269, or TNFRSF13A, is a type III transmembrane glycoprotein involved in B-cell survival and development. It is encoded by the TNFRSF17 gene located on the short arm of chromosome 16 (16p13.13). The gene is 2.92 kb in length and consists of three exons and two introns.
The BCMA protein is primarily composed of:
- Extracellular Domain (ECD): 54 amino acids.
- Transmembrane Domain (TM): 23 amino acids.
- Intracellular Domain (ICD): 107 amino acids.
A soluble form of BCMA (sBCMA) can also be produced through γ-secretase cleavage, retaining the extracellular domain and part of the transmembrane region.
Clinical drug progress
1. Multiple Myeloma
On August 27, 2024, Legend Biotech’s BCMA CAR-T product, Carvykti® (generic name: ciltacabtagene autoleucel injection), received approval from the National Medical Products Administration (NMPA) for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM). Previously, under Johnson & Johnson’s leadership, Carvykti had already been introduced in the United States, the European Union, and Japan, with approvals for second-line treatment in both the US and the EU, benefiting numerous multiple myeloma (MM) patients.
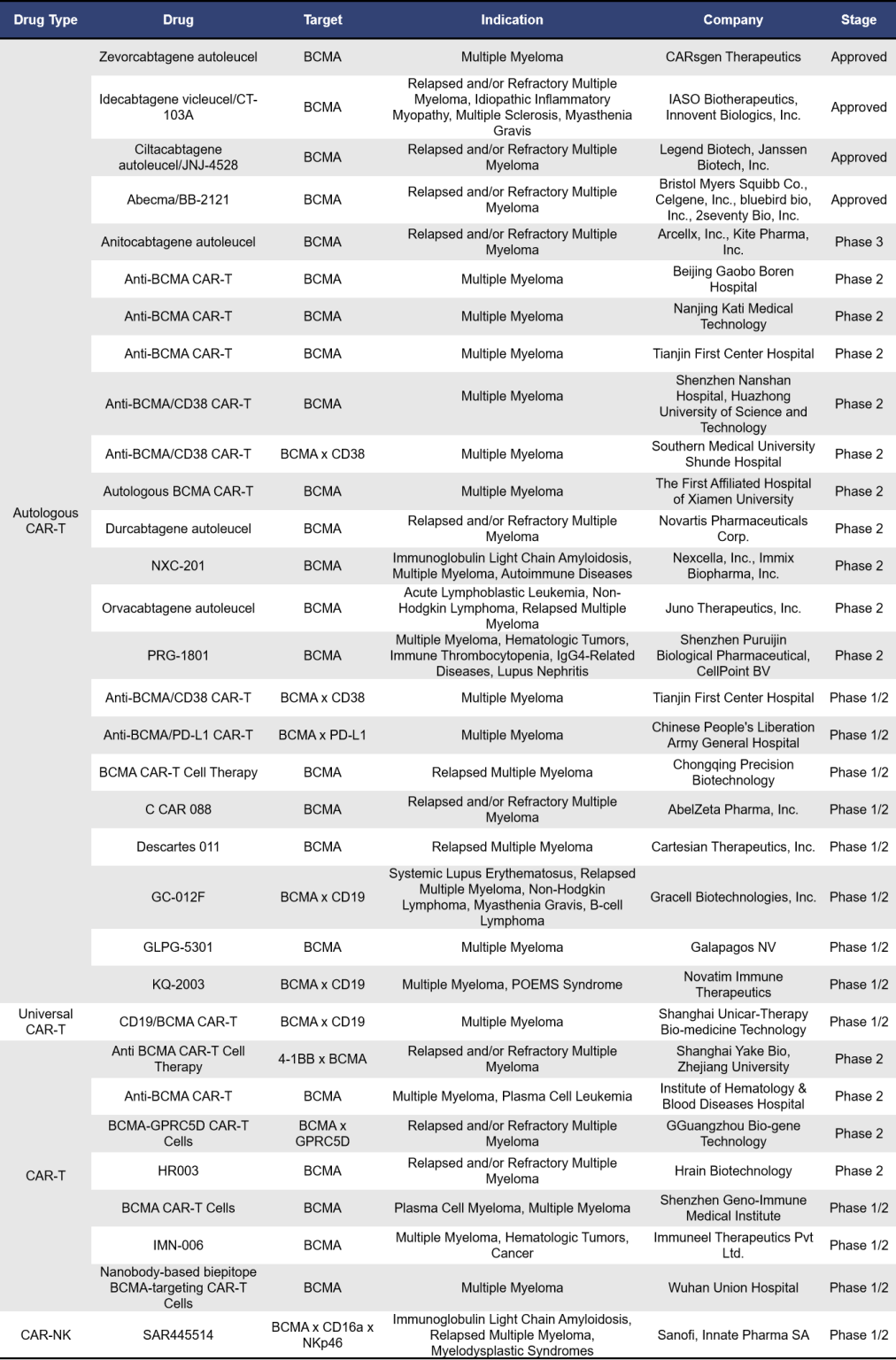
- BCMA CAR-T Therapy
BCMA-targeted CAR T cell therapies can be categorized into autologous BCMA, universal BCMA, allogeneic BCMA, and CAR-NK therapies. Currently, there are 77 such therapies either on the market or in clinical development. Of these, 49 are in early clinical phases (phase 1) or clinical phase 1. The table below highlights information on BCMA CAR-T therapies that are approved, in clinical phase 1/2, phase 2, and phase 3.
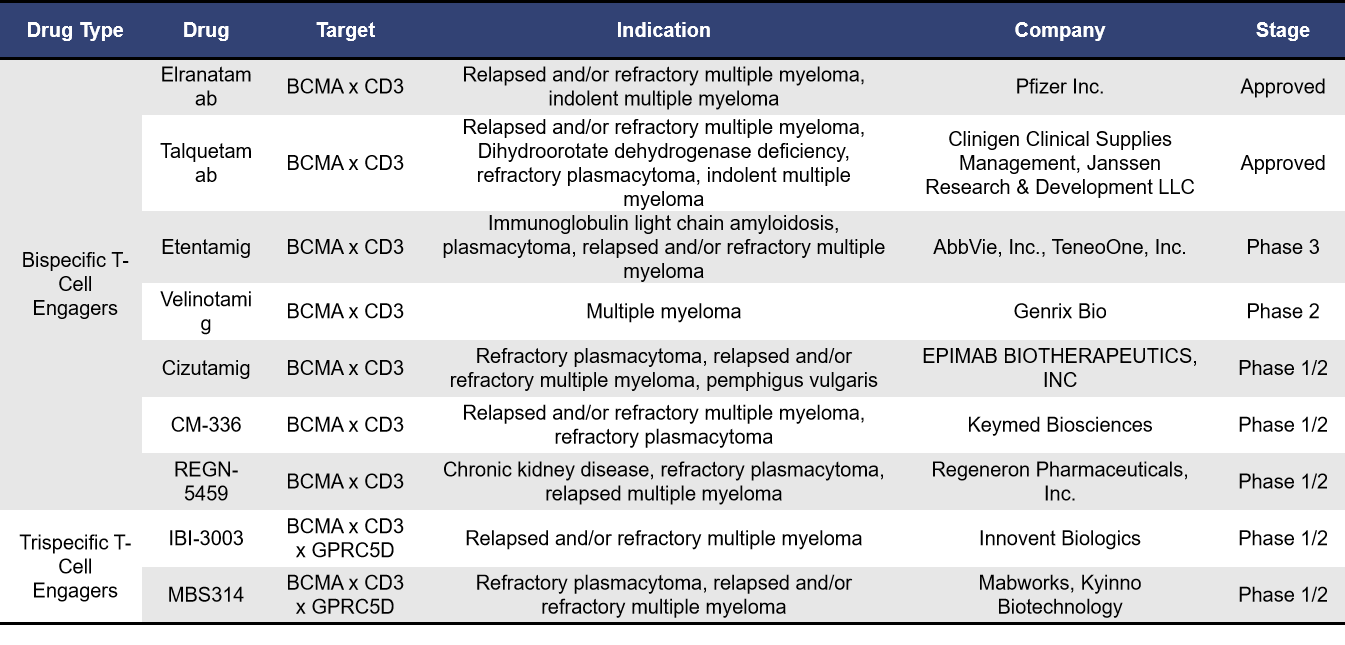
- BCMA-Targeted T-Cell Engagers
Currently, BCMA-targeted T-cell engagers globally fall into two main categories: bispecific T-cell engagers and trispecific T-cell engagers. According to incomplete statistics, there are 2 BCMA-targeted T-cell engagers already on the market and 15 in clinical development. Of these, 8 are in phase 1 trials. The table below highlights information on T-cell engagers that are either approved or in clinical phases 1/2, phase 2, and phase 3.
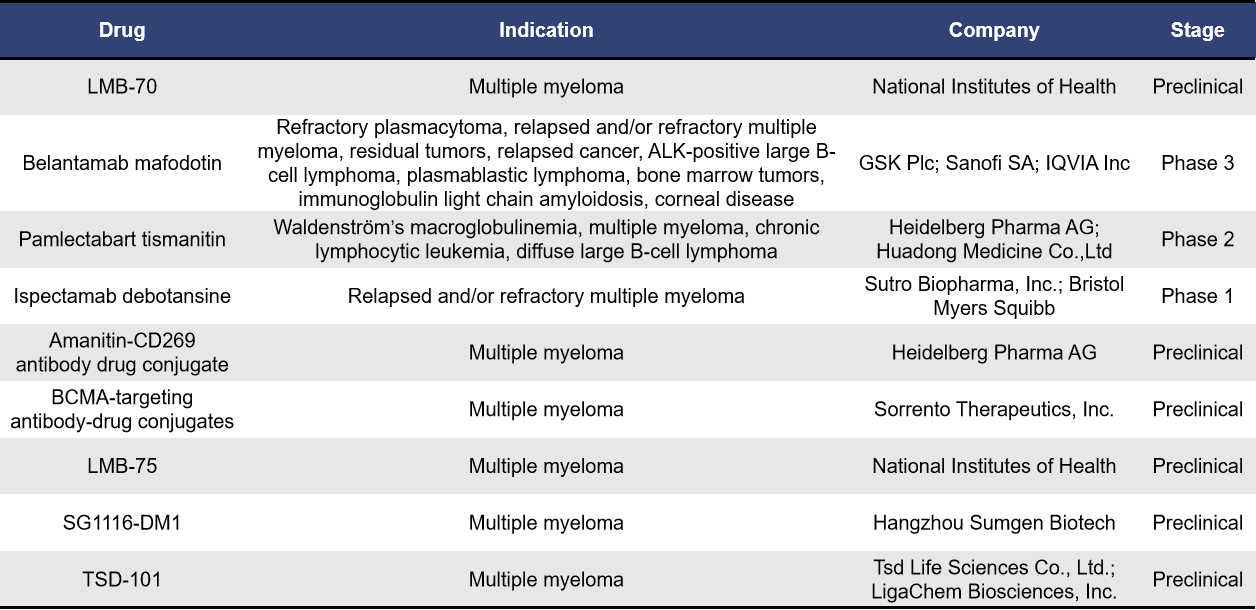
- BCMA-Targeted ADCs
Antibody-drug conjugates (ADCs) consist of three main components: a monoclonal antibody, a linker, and a cytotoxic agent. BCMA-targeted ADCs combine anti-BCMA antibodies with cytotoxic agents. The anti-BCMA antibody targets and binds to BCMA on the surface of multiple myeloma (MM) cells, leading to internalization of the ADC into the MM cells. Once inside, the ADC is degraded in the lysosomes, releasing the cytotoxic agent to exert its effect. According to incomplete statistics, there are currently 9 BCMA-targeted ADCs in development for multiple myeloma. Of these, 6 are in the preclinical stage, while one each is in clinical phases 1, 2, and 3.
2. Autoimmune Diseases
On May 10, 2024, a research team led by Wang Wei from the Department of Neurology at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, published a study in Science Immunology titled “Single-cell analysis of anti-BCMA CAR T cell therapy in patients with central nervous system autoimmunity.” The study reveals the mechanisms by which anti-BCMA CAR-T cell therapy affects relapsed/refractory neuromyelitis optica spectrum disorder (NMOSD). Most B cells in cerebrospinal fluid (CSF) are central nervous system (CNS) resident B cells, making them potential targets for CNS autoimmune diseases.
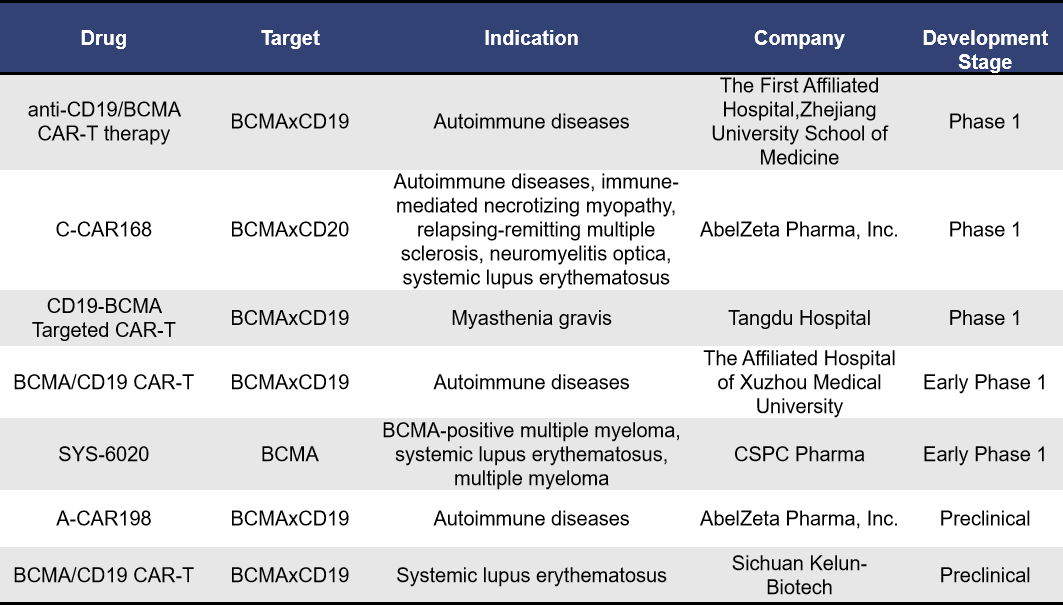
- Targeting BCMA CAR-T Therapies
Currently, there are 7 BCMA-targeting CAR-T therapies under development for autoimmune diseases. Among these, 3 are in Phase 1 clinical trials, 1 is in early Phase 1 clinical trials, and 2 are in preclinical stages.
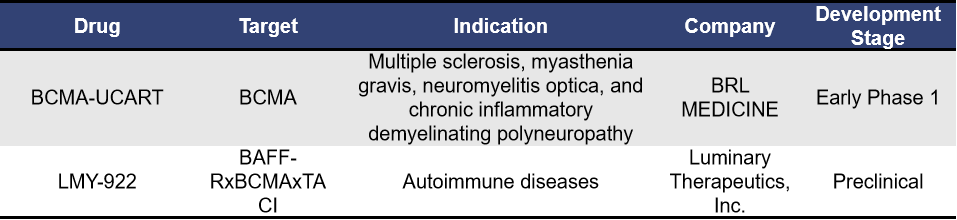
- Universal BCMA-Targeting CAR-T
Universal CAR-T cell therapy involves isolating T cells from healthy donors, which are then genetically edited or modified without genetic editing and expanded in vitro. The final product can be administered to multiple patients. Currently, for autoimmune diseases, there are mainly 2 universal BCMA-targeting CAR-T therapies, with BCMA-UCART from Shanghai Bangyao Biotechnology being a representative example.
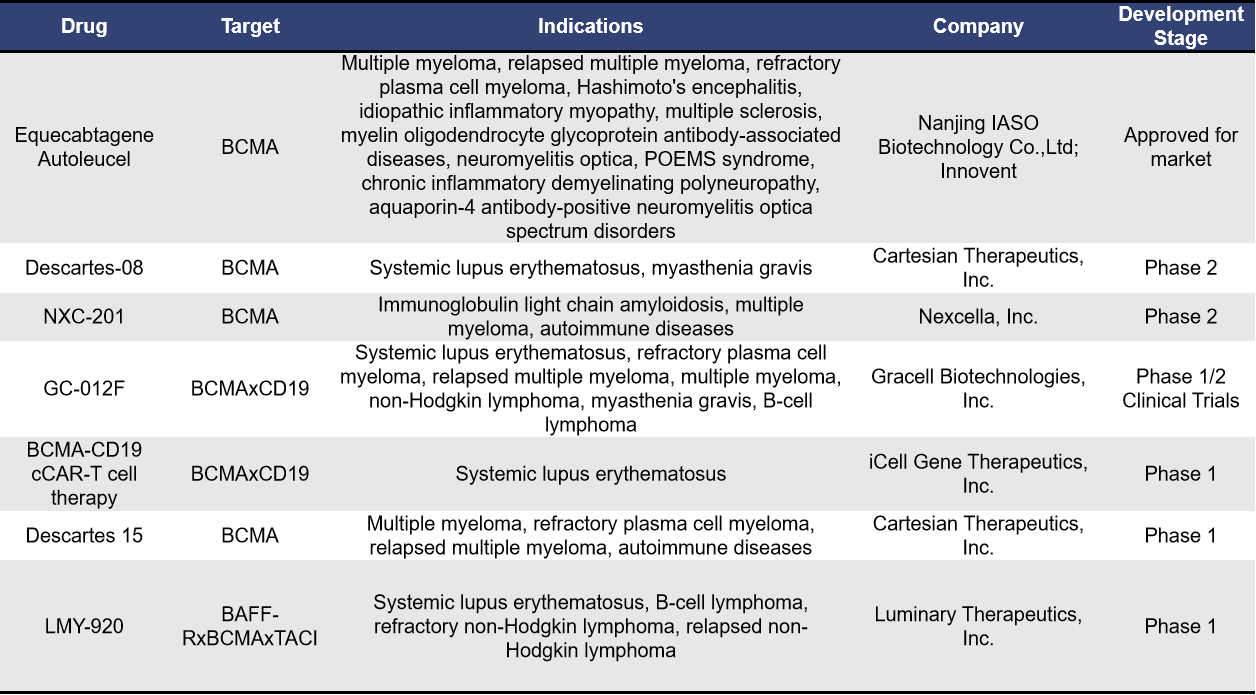
- BCMA-Targeted Autologous CAR-T
Autologous CAR-T, as the name suggests, involves using T cells derived from the patient’s own body. Currently, there are 7 BCMA-targeted autologous CAR-T therapies for autoimmune diseases. Among them, Descartes-08 and NXC-201 are progressing rapidly in clinical research. Although Equecabtagene Autoleucel has already been approved, its clinical research for autoimmune diseases is still in the Phase 1 stage (NCT04561557).
- Other therapies targeting BCMA
In addition to universal CAR-T and autologous CAR-T therapies, other targeted BCMA therapies in development for autoimmune diseases include CAR-NK cells, monoclonal antibodies, and antibody fusion proteins.