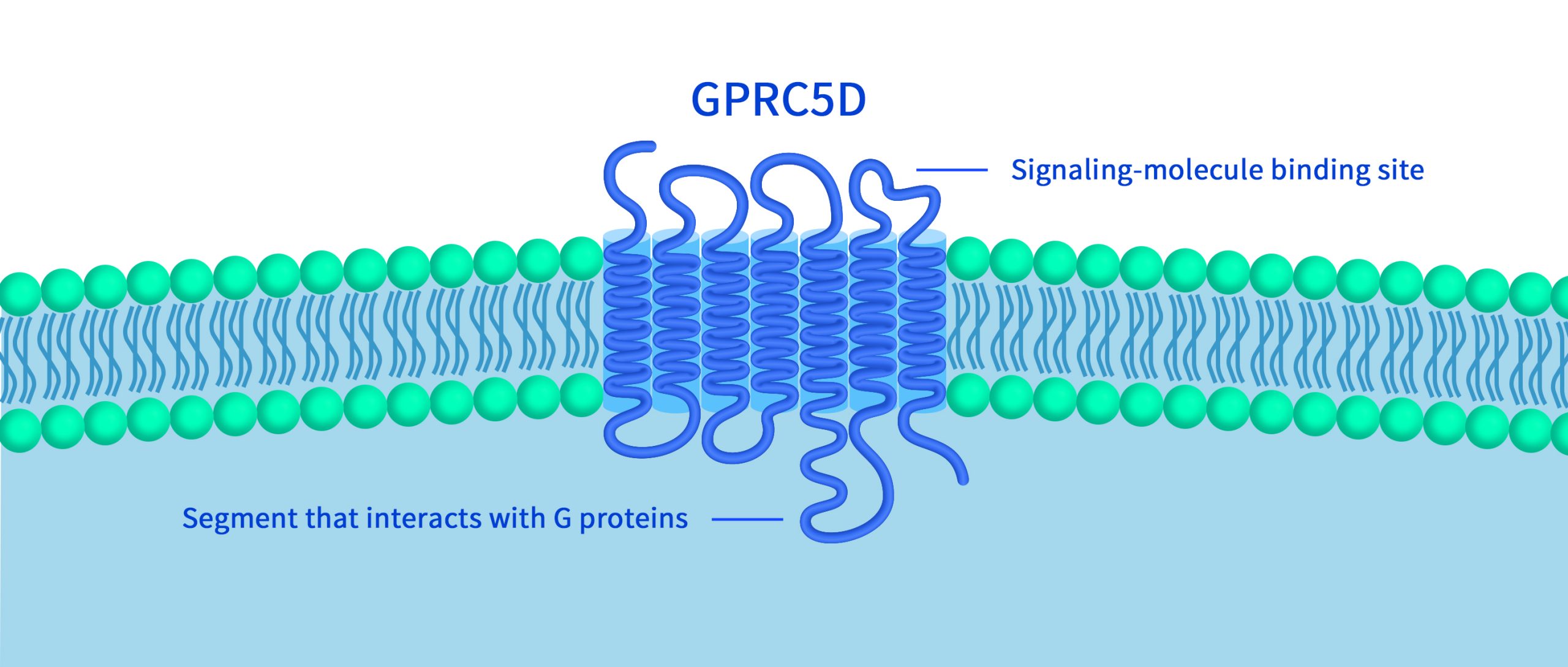
GPRC5D, identified as a G protein-coupled orphan receptor, demonstrates limited expression in normal tissues. However, it stands out with specific high expression in multiple myeloma (MM) cells, independent of BCMA. This unique expression pattern positions GPRC5D as a highly promising target for multiple myeloma treatment, following in the footsteps of BCMA (More information about GPRC5D). At the recently concluded 65th American Society of Hematology (ASH) annual meeting in late 2023, several clinical trial results regarding GPRC5D targeted biologics were unveiled. This overview delves into the clinical and pre-clinical research progress of GPRC5D dual-targeted therapies and CAR-T cell therapies.
1. Bispecific Antibodies
1.1 TALVEYTM (talquetamab-tgvs)
TALVEY™, also known as talquetamab-tgvs, is a GPRC5D x CD3 bispecific antibody developed by Johnson & Johnson, representing the most rapidly advancing antibody drug among all GPRC5D-targeted therapies. At the 2023 ASH conference, Johnson & Johnson unveiled various clinical research data for Talquetamab.
- 3377 Updated Results of Talquetamab, a GPRC5D×CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Multiple Myeloma with Prior Exposure to T-Cell Redirecting Therapies: Results of the Phase 1/2 MonumenTAL-1 Study
Previous results from the Phase 1/2 MonumenTAL-1 study (NCT03399799/NCT04634552) indicated that Talquetamab provides deep and durable responses in patients with relapsed/refractory multiple myeloma (RRMM). The overall response rates (ORR), exceeded 71% for 288 patients who had not received T-cell redirecting therapy (TCR), and 65% for 51 patients who had undergone TCR treatment. The latest updates include a total of 70 RRMM patients previously treated with TCR. The results show that Talquetamab achieved an ORR of 72.9% in patients who had prior BCMA CAR-T cell therapy, with a median duration of response (mDOR) exceeding 1 year. The ORR was 52.2% in patients previously treated with BCMA BsAb, and 75% in patients who had received BCMA ADC and TCR treatments [1].
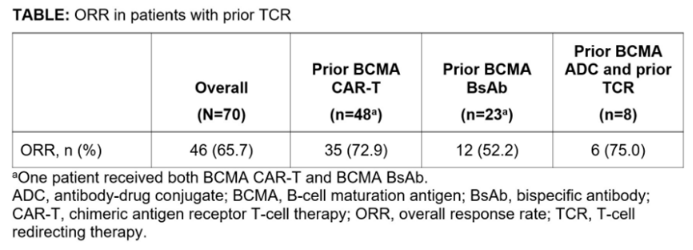
Data Source [1]
Regarding the Phase 1/2 MonumenTAL-1 study (NCT03399799/NCT04634552), this conference also presented reports on the Efficacy and safety of less frequent/Lower Intensity Dosing of Talquetamab in patients with Relapsed/Refractory Multiple Myeloma: Results from the Phase ½ MonumenTAL-1 study. (ID: 1010). Additionally, insights into the Mechanisms of Resistance and Relapse with Talquetamab in RRMM patients from the Phase 1/2 MonumenTAL-1 Study were discussed (ID: 1933).
- 1014 Talquetamab + Pomalidomide in Patients with Relapsed/Refractory Multiple Myeloma: Safety and Preliminary Efficacy Results from the Phase 1b MonumenTAL-2 Study
MonumenTAL-2 (NCT05050097) is a Phase 1b study investigating the combination of Talquetamab with the anti-myeloma drug Pomalidomide (Pom) in patients with multiple myeloma (MM). In this presentation, Johnson & Johnson showcased the preliminary efficacy and safety results of Talquetamab + Pom in the MonumenTAL-2 study. The findings reveal that the combination of Talquetamab and Pomalidomide induces rapid and profound responses in patients with RRMM or those who have received two or more prior lines of treatment (LOT). The ORR for the QW and Q2W cohorts were 86.7% and 83.3%, respectively, with complete response (CR) rates of 60% and 44.4%, and very good partial response (VGPR) rates of 86.7% and 77.8%. The median time to first response was 1.0 month for the QW cohort and 1.3 months for the Q2W cohort. The 6-month progression-free survival (PFS) rates for the two cohorts were 93.3% and 88.9% [2].
1.2 Lbl-034
4672 Lbl-034, a Highly Differentiated T-Cell Engaging Bispecific Antibody Targeting GPRC5D for the Treatment of Relapsed or Refractory Multiple Myeloma
Lbl-034 is a humanized IgG1 subtype asymmetric bispecific antibody developed independently by Nanjing VivaZome Bio targeting GPRC5D and CD3. It aims to execute targeted attacks on both GPRC5D and CD3. The unique molecular design of LBL-034 allows it to bind with high affinity specifically to tumor cells expressing GPRC5D while simultaneously reducing the risk of non-specific activation of T cells, thereby enhancing anti-tumor efficacy and reducing potential immune toxicity risks. Preclinical results disclosed by VivaZome at the 2023 ASH meeting show that Lbl-034 can induce potent T-cell-dependent cytotoxicity against GPRC5D, increase the expression of activation markers and cytokine release, enhance anti-tumor activity, while reducing the risk of CD3-induced Cytokine Release Syndrome (CRS), demonstrating good anti-tumor activity.
Currently, Lbl-034 has received clinical trial approval from the FDA and NMPA. The Phase I clinical trial (NCT06049290) is currently underway in China. This is a single-arm, open-label, multicenter, dose-escalation, and expansion first-in-human Phase I/II clinical study. Its purpose is to validate the safety and tolerability of Lbl-034 in patients with RRMM, determine the recommended dose for Phase I/II clinical trials, and evaluate its therapeutic effects.
1.3 RG6234
1948 Co-Expression of GPRC5D, FcRH5 and BCMA Suggests That Targeting More Than One Cell Surface Marker May be a Viable Strategy in Relapsed/Refractory Multiple Myeloma (RRMM): Biomarker Results from the Phase I Study of Forimtamig, a GPRC5DxCD3 Bispecific Antibody
RG6234, also known as Forimtamig, is a novel 2:1 structured bispecific monoclonal antibody developed by Roche, targeting GPRC5DxCD3. It comprises two protein domains binding to the target and one binding to CD3. Currently, it is in the Phase I clinical stage globally.
Roche presented exploratory biomarker results from the Phase I study of Forimtamig at the 2023 ASH meeting. The study indicated that GPRC5D and FcRH5 were co-expressed on 75.4% of multiple myeloma plasma cells (MMPC). Additionally, co-expression was observed between GPRC5D and BCMA (median 31.9%) or BCMA and FcRH5 (median 25.3%). Rare occurrences of double-negative and triple-negative MMPCs were observed in most patients, with only 0.5-6% of MMPCs expressing only one or none of the three targets. The co-expression of GPRC5D, FcRH5, and BCMA suggests that targeting multiple MM surface markers might result in dual/triple hits on individual tumor cells. This approach could facilitate rapid and profound tumor clearance, achieving sustained responses in patients with relapsed/refractory multiple myeloma (RRMM), while potentially avoiding clone selection.
RG6234, with its unique design targeting multiple cell surface markers, represents a promising strategy in the evolving landscape of RRMM treatment. The findings from the Phase I study shed light on the potential of Forimtamig in delivering a comprehensive and effective therapeutic response by addressing the heterogeneity of MMPC surface marker expression. Further clinical development and exploration will be crucial to validate and expand upon these promising preliminary results.
2. Trispecific Antibody
456 Characterization of JNJ-79635322, a Novel BCMAxGPRC5DxCD3 T-Cell Redirecting Trispecific Antibody, for the Treatment of Multiple Myeloma
JNJ-79635322, developed by Johnson & Johnson, is a trispecific antibody targeting BCMA/GPRC5D/CD3. It consists of binding domains against CD3, BCMA, and GPRC5D. This trispecific antibody, utilizing the CD3-arm modified T-cell engaging antibody, facilitates dual antigen recognition on plasma cells. This approach enhances affinity for tumor binding, potentially enabling effective clearance of malignant clone populations and preventing tumor antigen loss-mediated resistance. Currently, it is in the Phase I clinical development stage globally.
Johnson & Johnson presented preclinical trial results for JNJ-79635322 at the 2023 ASH meeting. The findings suggest that JNJ-79635322 induces potent cytotoxicity in various myeloma cell lines in vitro, accompanied by T-cell activation. Importantly, JNJ-79635322 effectively eliminates cells expressing both targets (BCMA and GPRC5D) as well as those expressing a single target (BCMA or GPRC5D). Furthermore, in vivo, JNJ-79635322 demonstrates effective anti-tumor activity in a mouse MM xenograft prevention model (expressing a single target clone H929 cells) and two tumor regression models (RPMI 8226 and MM.1S cells) [3].
Currently, a Phase I dose-escalation study (NCT05652335) is underway to investigate the treatment of myeloma patients with JNJ-79635322. The results from this study will provide valuable insights into the safety, tolerability, and potential therapeutic efficacy of this novel trispecific antibody in the clinical setting.
3. CAR-T cell therapy
3.1 BMS-986393
219 BMS-986393 (CC-95266), a G Protein–Coupled Receptor Class C Group 5 Member D (GPRC5D)–Targeted Chimeric Antigen Receptor (CAR) T-Cell Therapy for Relapsed/Refractory Multiple Myeloma (RRMM): Updated Results from a Phase 1 Study
BMS-986393 (CC-95266) is an autologous CAR T-cell therapy developed by Bristol Myers Squibb (“BMS”) targeting GPRC5D. It is currently in the highest clinical research stage, Phase I. BMS presented data from a first-in-human, multicenter, open-label Phase I study at the 2023 ASH conference, assessing the efficacy of BMS-986393 in patients with RRMM who had received three or more prior therapies. The primary objectives of the study were to determine the safety, tolerability, maximum tolerated dose, and/or recommended Phase II dose (RP2D) of BMS-986393, with secondary objectives including the assessment of preliminary efficacy.
The disclosed results indicated that in evaluable patients, the overall response rate (ORR) across different doses was 86% (55/64), and in patients previously treated with BCMA-targeted therapies (including CAR-T cells), the ORR was 75% (21/28). The CR rate was 38% (24/64). In patients with a history of refractory BCMA-targeted therapy, the ORR was 85% (11/13), with a CR rate of 46% (6/13). The median follow-up time for all treated patients was 5.9 months (range, 0.0-24.0).
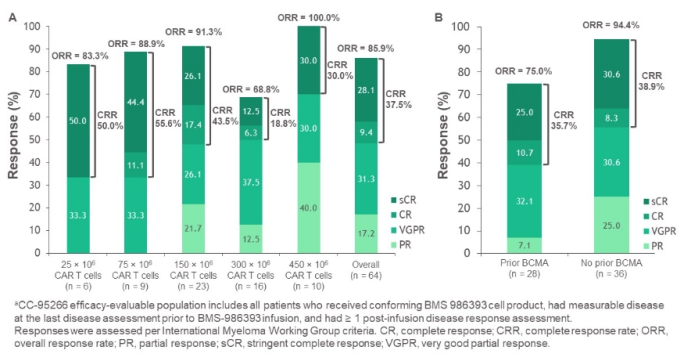
Data Source [4]
In this first-in-human study, BMS-986393 demonstrated manageable safety and sustained deep responses across all tested dose levels. Cytokine Release Syndrome (CRS) and Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) were mostly of lower grades, with an increase in ≥3-grade events observed at 300×10^6 and 450×10^6 CAR-T cell doses. A minority of patients experienced Grade 1/2 off-target treatment-related adverse events (TRAEs).
3.2 DIMABIO&Guangzhou Bio-gene& 920th Hospital GPRC5D CAR T Cell Therapy
3472 Safety and Efficacy of GPRC5D CAR T Cell Therapy in Relapsed/Refractory Multiple Myeloma Patients
GPRC5D CAR-T Cell Therapy, jointly developed by Wuhan DIMA Biotech, Guangzhou Bio-gene Technology and 920th Hospital of Joint Logistics Support Force, is a second-generation CAR-T cell therapy targeting GPRC5D. It consists of a single-chain variable fragment (scFv) derived from a humanized rabbit monoclonal antibody targeting GPRC5D, a 4-1BB co-stimulatory domain, and a CD3ze signaling domain. The current global clinical development stage is Exploratory Clinical IIT Study. Dr. Donghui Ma, CEO of DIMA Biotech, presented the clinical trial results at the 2023 ASH conference.
The NCT05739188 trial is a single-center, open-label, single-arm Phase I study designed to assess the efficacy and safety of anti-GPRC5D CAR-T cell therapy in RRMM subjects. As of now, a total of 7 RRMM patients have been enrolled in the trial, including 3 patients previously treated with BCMA CAR-T therapy. All patients received autologous CAR T-cell infusions and underwent clinical assessments between February 7, 2023, and June 26, 2023. The overall response rate was 85.7% (6/7), with 5 cases achieving complete response (CR), 1 case achieving partial response (PR), and 1 case showing no response (NR). The NR patient had previously shown no response to BCMA CAR-T therapy, and FACS analysis confirmed the absence of BCMA and GPRC5D on tumor cells. As of the current results, GPRC5D CAR T cell therapy demonstrates good clinical efficacy and tolerable safety in RRMM patients. The clinical trial is still actively recruiting patients.
4. DIMA Biotechnology LTD: Advancing Biomedical Solutions
DIMA Biotechnology LTD stands as a leading biotechnology company committed to delivering cutting-edge products and services for preclinical research, focusing on druggable targets. Our pioneering platforms cover the spectrum of functional membrane protein development, single B-cell lead antibody discovery, antibody engineering, and rigorous functional validation.
Our Offerings:
- A diverse array of products and services for GPRC5D protein, featuring active proteins, flow-validated monoclonal antibodies, and Benchmark reference antibodies.
- Specialized services include antibody humanization, affinity maturation, and removal of PTM risk sites, among others.
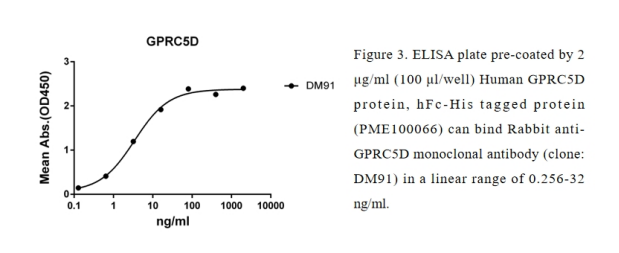
As a testament to our commitment to GPRC5D drug development, DIMA is pleased to offer a complimentary sample of our flagship anti-GPRC5D rabbit monoclonal antibody (DM91, Cat# DME100091) & Anti-GPRC5D antibody(DM61); Rabbit mAb (DME100061).
Explore our complete range of GPRC5D products and take a step towards groundbreaking biomedical advancements. Click here to learn more>>
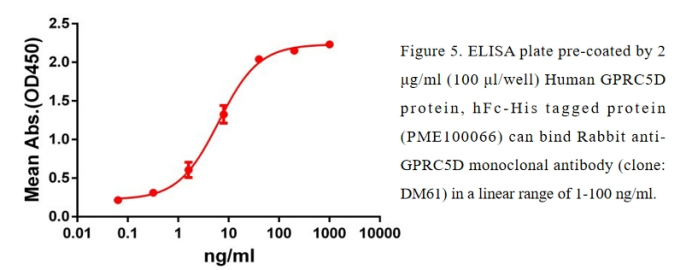
ELISA application
FC application
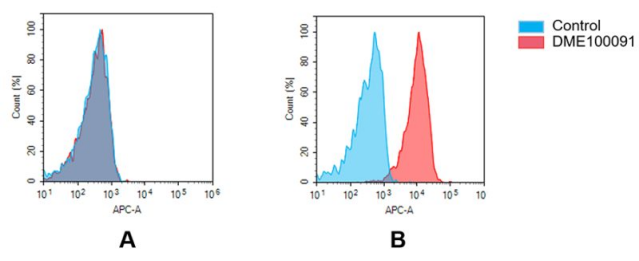
Figure 4. Flow cytometry analysis of antigen binding of rabbit anti-human GPRC5D mAb (DME100091). (A) DME100091 does not bind to Jurkat cells that do not express GPRC5D. (B) A clear peak shift of DME100091 was seen compared to the control when incubated with GPRC5D-expressing MM.1S cells, indicating strong binding of DME100091 to GPRC5D. Antibodies were incubated at 5 µg/mL.
ELISA application
IHC application
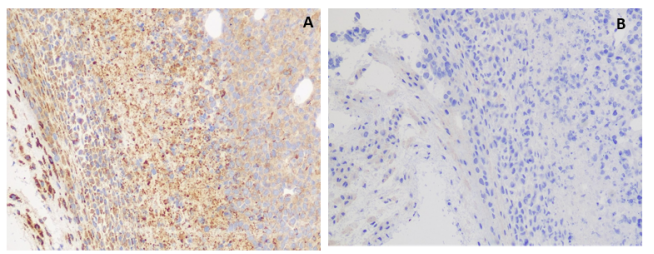
Figure 6. IHC staining of H929 cells A. with rabbit anti-GPRC5D mAb (Cat# DME100061) at 5.9 ug/ml. B. with another anti-GPRC5D mAb (Cat# DME100090) at 8.8 ug/ml. DME100061 can specifically react with GPRC5D in IHC application
- Free Sample Application
- Period: January 1, 2024 – January 31, 2024
- Target Audience: End-users
- Details: This offer cannot be combined with other promotions. The final interpretation of this event belongs to DIMA Biotechnology LTD.
- Other GPRC5D Product Trials: DIMA Biotechnology has independently developed various GPRC5D-related antibody products. If you require products labeled with PE or Biotin, please fill in your needs in the form. DIMA staff will contact you within 1 working day after submission.
- Application Method: Please send a message to info@dimabio.com or leave a message on CONTACT US.
Reference:
[1]Andrzej J Jakubowiak,et al. 2023 ASH. Poster 3377.
[2]Jeffrey V Matous, et al. 2023 ASH. Oral 1014.
[3]Ram Pillarisetti, et al. 2023 ASH. Oral 456.
[4]Susan Bal, MD, et al. 2023 ASH. Oral 219.