According to a blog released by market intelligence provider Future Market Insights, Inc. (FMI) on September 12 this year, the global market for systemic lupus erythematosus (SLE) drugs is showing significant growth, with surprising acceleration. FMI predicts that the SLE drug market will experience robust expansion in the coming years. In 2020, the market was valued at $183.3 billion and is expected to reach an astonishing $329.18 billion by 2032, with a compound annual growth rate (CAGR) of 5%. The report indicates that the growth of the SLE market can be attributed to advancements in biologics and targeted therapies, a deeper understanding of disease mechanisms, clinical trials and research initiatives, as well as government plans aimed at treating rare diseases. So, which pharmaceutical companies are investing in SLE drugs? Before we dive into that, let’s briefly explore the background of SLE.
1. Overview of SLE
Systemic lupus erythematosus (SLE) is an autoimmune disease that affects multiple systems and organs, characterized by the production of autoantibodies against cellular nuclear components, leading to a wide range of clinical manifestations. Although the exact pathology of SLE remains unclear, its pathogenesis is complex, involving the interplay of environmental factors, genetics, epigenetics, and immune responses. Among these, immune dysregulation is considered the primary mechanism driving the disease.
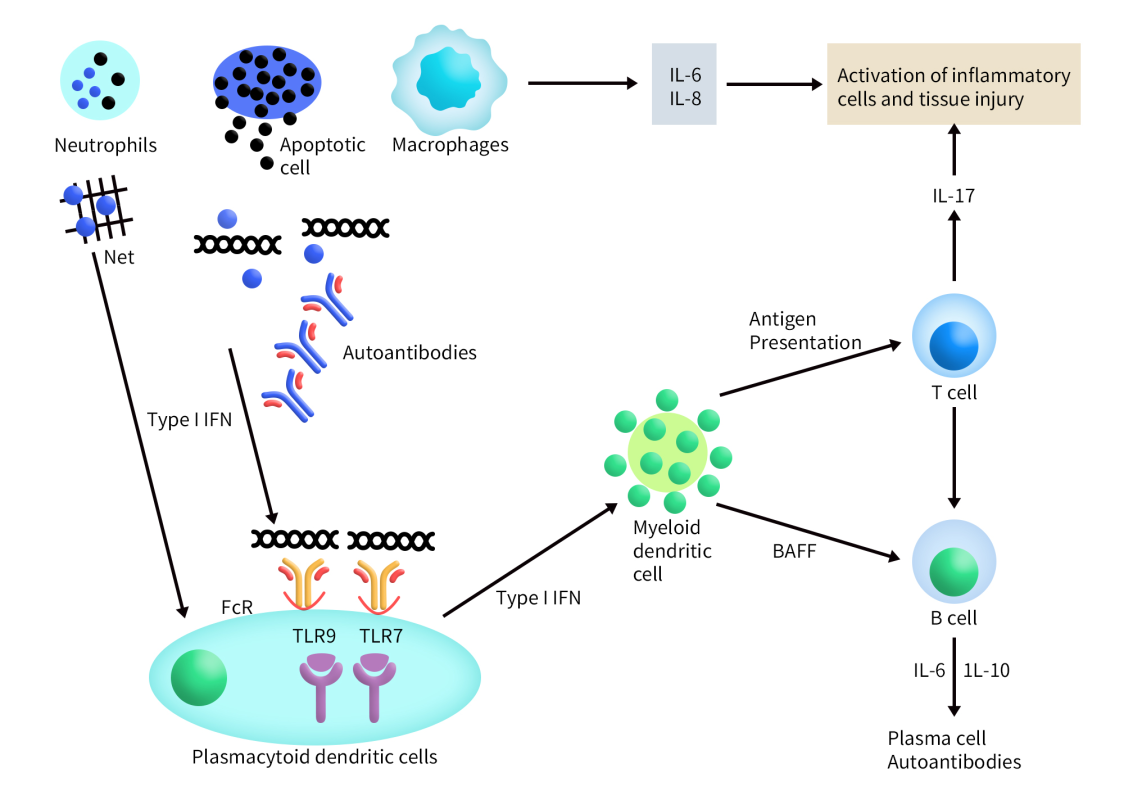
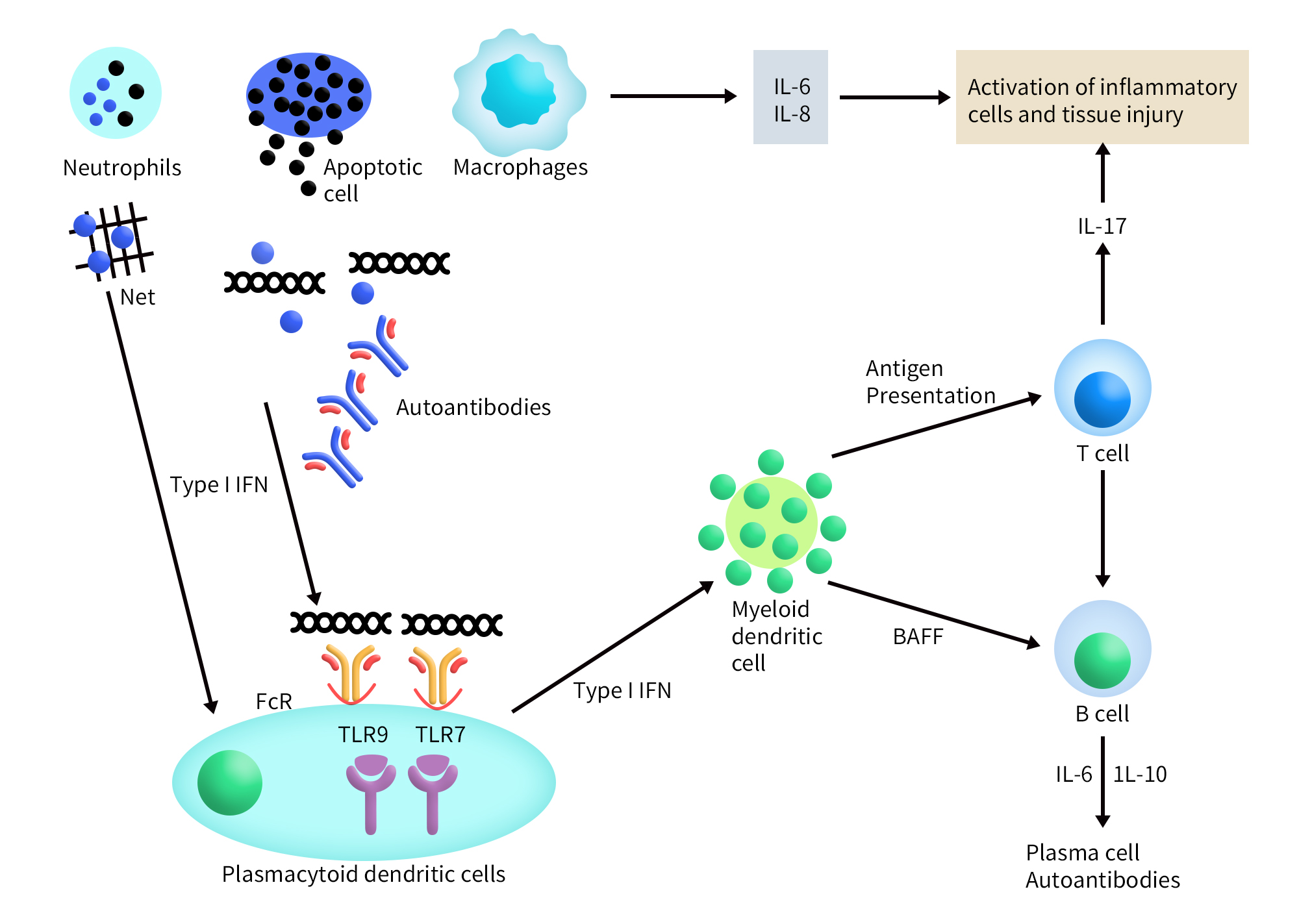
Figure 1. Immunobiology of systemic lupus erythematosus [1]
The immune dysregulation in systemic lupus erythematosus (SLE) primarily involves interactions between innate/adaptive immune factors and cells within the microenvironment, triggering immune responses. Neutrophils play a crucial role as a significant triggering factor in SLE. Elevated levels of reactive oxygen species (ROS) and enhanced IL-18 receptor expression lead to neutrophil dysregulation. This dysregulation is associated with increased neutrophil extracellular traps (NETs), which correlate with heightened IgG2 secretion by B cells in lupus [3].
Furthermore, neutrophil degranulation increases the secretion of pro-inflammatory cytokines, such as IFN-γ, exacerbating lupus symptoms [4]. Innate immune receptors, like Toll-like receptors (TLRs), recognize pathogen-associated molecular patterns (PAMPs) and can activate the immune system by linking innate and adaptive responses. TLRs 1, 2, 4, 5, and 6, which respond to bacterial or fungal PAMPs, are located on the cell surface, while TLRs 3, 7, 8, and 9, which respond to nucleic acids (single-stranded/double-stranded RNA or DNA), are found on endosomal membranes [5].
Among these, TLR7 and TLR9 are closely related to the pathogenesis of SLE. They mediate the production of IFNα by plasmacytoid dendritic cells (pDCs) after being activated by circulating immune complexes containing self-nucleic acid components [6]. TLR7 enhances the follicular B cell response in germinal centers, promoting the production of autoantibodies, while TLR9 appears to limit the stimulatory activity of TLR7, suggesting a protective role in lupus pathology [7]. Additionally, platelets contain functional TLR7, which is responsible for platelet activation and may contribute to platelet-leukocyte aggregation, potentially initiating immune responses following viral attacks [8].
Research has also shown that other TLRs, such as TLR2 and TLR4, are highly expressed in the saliva of SLE patients. However, the presence of chronic periodontitis in lupus patients suggests that their expression levels may be lower in this context [9].
2. Global Progress in SLE Drug Research
Currently, there are approximately 478 drugs targeting systemic lupus erythematosus (SLE) worldwide. Among these, the largest group comprises preclinical drugs, totaling 93. There are 72 drugs that have been approved for market use, while 64 are in Phase II clinical trials and 63 are in Phase I trials. Additionally, there are 102 drugs that are either stagnant or have been terminated. Overall, this pharmaceutical sector shows a robust pipeline of drugs in the development stage, with many having already gained approval, indicating significant potential for growth.
When analyzing the landscape of companies involved in SLE drug development, it is notable that large pharmaceutical firms like Pfizer Inc., Novartis AG, and Bristol Myers Squibb Co. have a significant number of drugs entering clinical stages, but they have relatively few products that have received market approval. In contrast, mid-sized and smaller companies, such as Jiangsu Hengrui Pharmaceuticals, Amgen Inc., and AstraZeneca PLC, may have fewer drugs in clinical trials, but they boast a richer portfolio of approved products.
From a target perspective, the clinical drugs predominantly focus on CD19, followed by BTK and TYK2. This highlights the strategic targeting of specific pathways in SLE treatment and showcases the clinical drugs and the companies developing them.
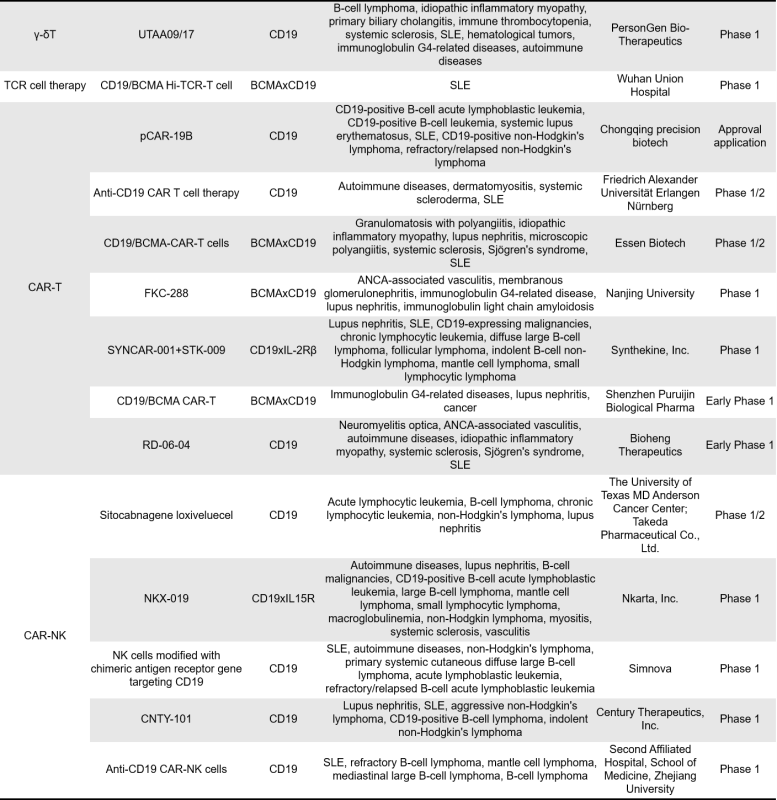
2.1 SLE Drugs Targeting CD19
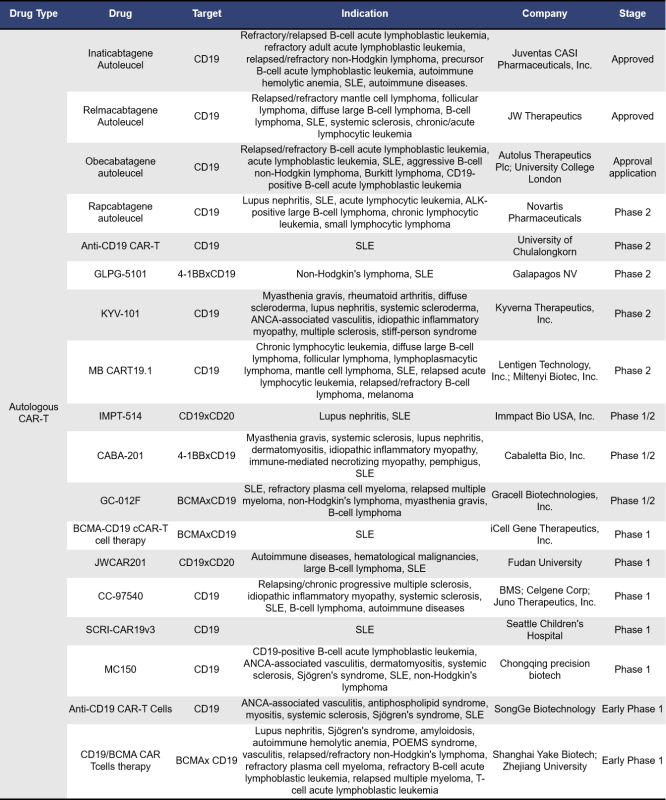
Currently, there are a total of 49 SLE drugs globally that target the CD19 pathway, comprising both approved and clinical-stage products. Notably, nearly half of these (23 drugs) are in Phase 1 clinical trials, while 6 drugs have been approved or are in the process of approval. Additionally, there are 6 drugs in Phase 2 and Phase 3 trials.
Among these, the largest group consists of single-target drugs specifically targeting CD19, totaling 30. Following this, there are 7 dual-target drugs that focus on both BCMA and CD19 (click here to learn more about BCMA research in autoimmune diseases). There are also two drugs targeting both CD19 and CD20, as well as CD19 and 4-1BB. Additionally, single drugs target combinations of CD19 and IL15R, CD19 and CD32B, CD19 and IL-2Rβ, and CD19 and PD-1. Furthermore, two drugs target three different pathways, specifically CD19, CD2, and CD3, and CD19, CD28, and CD3. Here is a detailed overview of some key drugs targeting CD19:
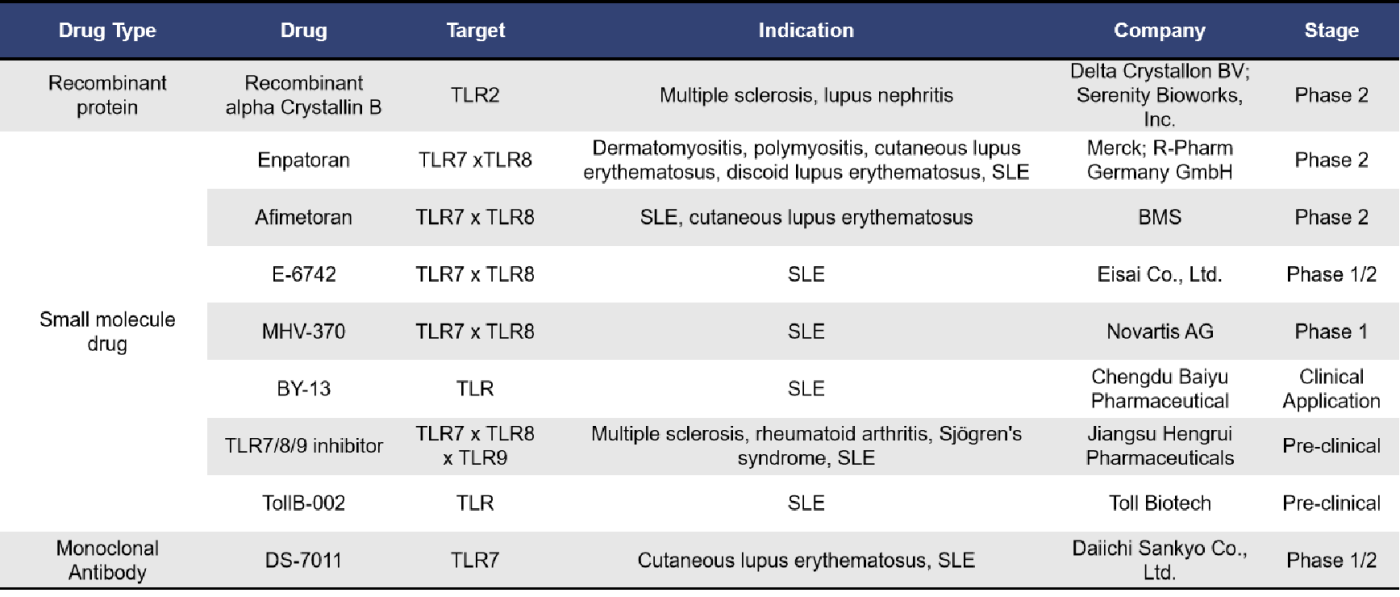
2.2 TLR-Targeted SLE Drugs
Currently, there are a total of 9 investigational drugs targeting Toll-like receptors (TLRs) for systemic lupus erythematosus (SLE) worldwide. Among these, 5 drugs specifically target both TLR7 and TLR8. The drug types include 1 recombinant protein, 7 small molecule drugs, and 1 monoclonal antibody. The fastest clinical research progress is seen with Delta Crystallon BV’s Recombinant Alpha Crystallin B, Merck’s Enpatoran, and Bristol Myers Squibb’s Afimetoran, all of which are in Phase 2 clinical trials.
Recombinant Alpha Crystallin B (also known as DC-TAB or SER-101) is a recombinant human HspB5 targeting TLR2. It induces a persistent tolerance pathway in myeloid cell populations and is currently being advanced clinically by Serenity Bioworks for the treatment of lupus nephritis.
Enpatoran is a TLR7/8 antagonist developed by Merck in Germany. It has been shown to specifically inhibit various ligands that activate TLR7/TLR8. Activation of TLR7 and TLR8 is known to stimulate antibody production, secretion of interferons (IFNs) and other cytokines, cell maturation, and other protective host mechanisms. Inhibiting their activation can reduce disease activity, severity of acute lupus flares, and corticosteroid requirements in patients with systemic lupus erythematosus (SLE) and cutaneous lupus erythematosus (CLE). Merck is conducting a Phase 2 clinical trial for enpatoran to evaluate its efficacy and safety in patients with cutaneous lupus erythematosus and systemic lupus erythematosus.
Afimetoran, developed by Bristol Myers Squibb (BMS), is another TLR7/8 antagonist with a mechanism of action similar to that of enpatoran. It is also currently in Phase 2 clinical trials.
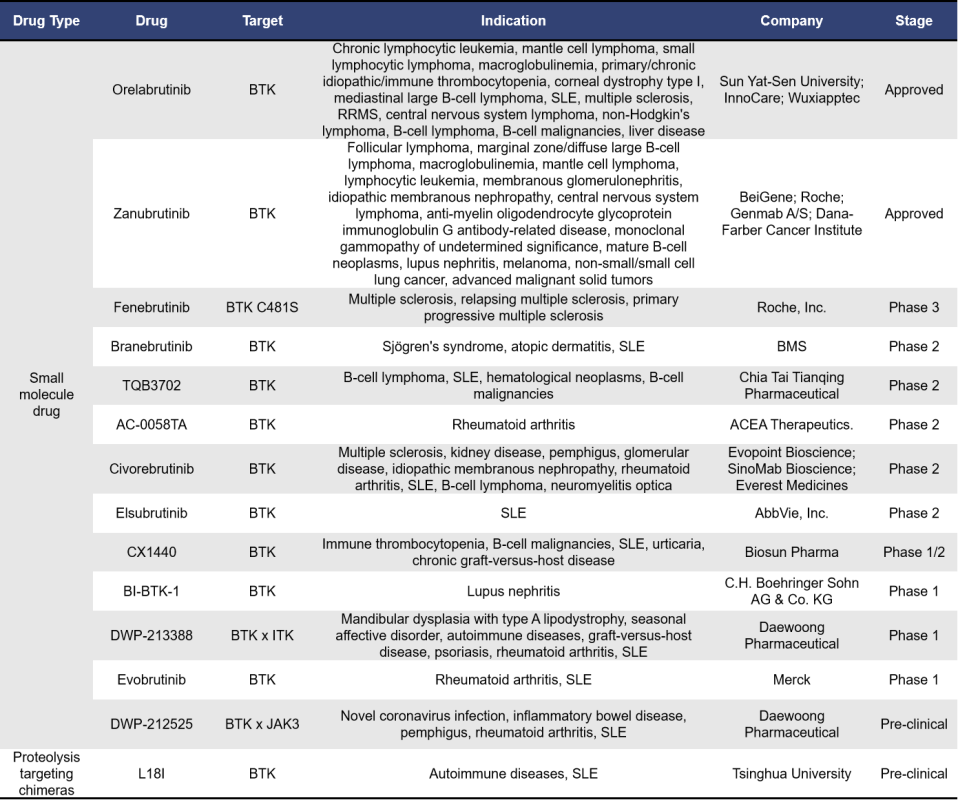
2.3 BTK-Targeted SLE Drugs
Bruton’s Tyrosine Kinase (BTK) is a key kinase in the B cell receptor signaling pathway, playing crucial roles in regulating B cell maturation, proliferation, differentiation, apoptosis, and migration. Abnormal activation of BTK can lead to hyperactive B cell receptor signaling, which is closely linked to the development and progression of B cell malignancies. Given this, BTK inhibitors have achieved significant success in the field of hematologic malignancies.
Since autoimmune diseases often involve abnormal selection and activation of autoreactive B cells, leading to subsequent production of autoantibodies, BTK represents a promising therapeutic target for autoimmune diseases, including systemic lupus erythematosus (SLE).
Currently, 14 BTK-targeted drugs are in development for SLE globally. Of these, 13 are small-molecule drugs and 1 is a proteolytic-targeting chimeric molecule. Among the small molecules, 2 have already been approved, 1 is in Phase 3 clinical trials, and 5 are in Phase 2. The proteolytic-targeting chimeric molecule is still in the preclinical research stage.
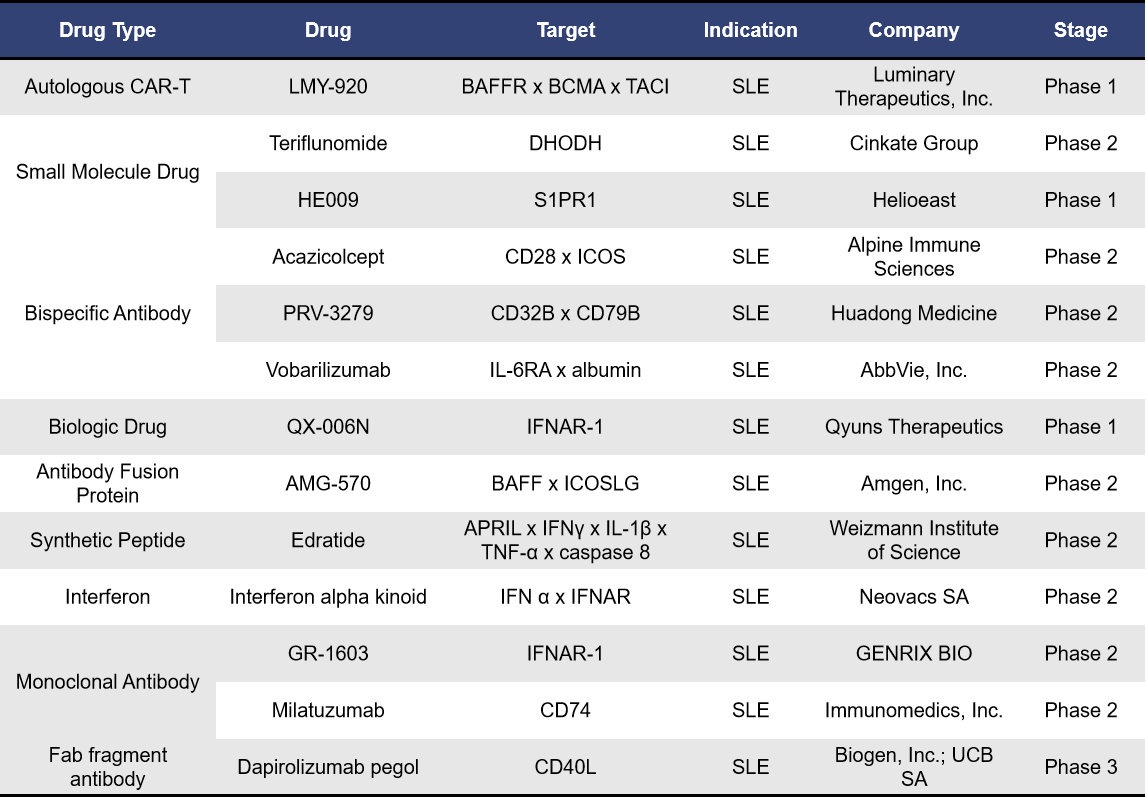
In addition to the prominent targets discussed earlier, several less common targets for SLE treatment include CD40L, CD74, DHODH, and S1PR1. Below are some clinical-stage drugs targeting these specific pathways, along with their respective research institutions.
3. DIMA Biotech: Advancing Autoimmune Drug Development with Ready-to-Use Lead Molecules
DIMA Biotech is a biotechnology company established in 2018, dedicated to providing products and services for biopharmaceutical companies. The company has developed over 5,000 ready-to-use lead antibody molecules targeting more than 400 popular drug targets. Each antibody possesses unique sequences and validation data.
In the coming years, DIMA aims to expand its portfolio to include ready-to-use antibodies for all druggable targets, thereby accelerating the preclinical development of antibody drugs for biopharmaceutical companies. This strategic focus positions DIMA as a key player in enhancing the efficiency and effectiveness of drug discovery processes in the autoimmune disease sector.
DIMA Biotech has developed several proprietary technology platforms, including:
- DiMPro™ Membrane Protein Expression Platform: Utilizes a mammalian cell expression system combined with advanced Nanodisc technology to produce and purify various full-length transmembrane proteins, including multiple GPCRs and ion channels.
- Single B Cell Monoclonal Antibody Development Platform: Successfully developed 5000+ lead antibodies for over 400 druggable targets, with IgG sequence information and validation data.
- DiLibrary™ Mammalian Cell Display-Based Antibody Engineering Platform: Designed for antibody humanization and affinity maturation, resulting in antibodies with enhanced druggability and affinity.
- DiTag™ ADC Antibody Detection Reagent: Effectively assesses the efficiency of ADC (Antibody-Drug Conjugate) antibodies.
These platforms collectively strengthen DIMA Biotech’s ability to support the development of innovative therapies in the biopharmaceutical industry.
References:
[1]Akhil, A., Bansal, R., Anupam, K. et al. Systemic lupus erythematosus: latest insight into etiopathogenesis. Rheumatol Int, 2023, 43, 1381-1393.
[2]Ma J et al. Elevated interleukin-18 receptor accessory protein mediates enhancement in reactive oxygen species production in neutrophils of systemic lupus erythematosus patients. Cells, 2021, 10(5):964
[3]Bertelli R et al. Neutrophil extracellular traps in systemic lupus erythematosus stimulate IgG2 production from B lymphocytes. Front Med, 2021, 8:635436.
[4]Liu Y, Kaplan MJ. Neutrophil dysregulation in the pathogenesis of systemic lupus erythematosus. Rheum Dis Clin, 2021, 47(3):317–333.
[5]Pan L et al. Immunological pathogenesis and treatment of systemic lupus erythematosus. World J Pediatr, 2020, 16(1):19–30.
[6]Nasser N, Kurban M, Abbas O. Plasmacytoid dendritic cells and type I interferons in flares of systemic lupus erythematosus triggered by COVID-19. Rheumatol Int, 2021, 41(5):1019–1020.
[7]Fillatreau S, Manfroi B, Dörner T. Toll-like receptor signalling in B cells during systemic lupus erythematosus. Nat Rev Rheumatol, 2021, 17(2):98–108
[8]Brilland B, Scherlinger M, Khoryati L, et al. Platelets and IgE: shaping the innate immune response in systemic lupus erythematosus. Clin Rev Allerg Immunol, 2019, 58:1–19.
Marques CP et al. Expression of Toll-like receptors 2 and 4 in the saliva of patients with systemic lupus erythematosus and chronic periodontitis. Clin Rheumatol, 2021, 40(7):2727–2734.