Introduction
Recent advancements in the field of nuclear medicine have highlighted the significance of B-Cell Maturation Antigen (BCMA) as a pivotal target for the diagnosis and treatment of Multiple Myeloma (MM). In a groundbreaking study published in the European Journal of Nuclear Medicine and Molecular Imaging, a collaborative effort by the molecular imaging team at the First Affiliated Hospital of Jinan University and the Chinese Academy of Medical Sciences has demonstrated the efficacy of the radiolabeled antibody [89Zr]Zr-DFO-BCMAh230430 in non-invasive PET imaging of MM. This research represents a significant step forward in the development of specific immuno-PET probes targeting BCMA, which is highly expressed in malignant plasma cells.
Background
Multiple Myeloma is the second most prevalent hematological malignancy, characterized by uncontrolled proliferation of monoclonal plasma cells within the bone marrow, leading to osteolytic bone lesions and organ damage. Despite advancements in treatment strategies such as autologous stem cell transplantation, high-dose therapy, and the introduction of novel agents including proteasome inhibitors and monoclonal antibodies, most MM patients eventually relapse, progressing to relapsed/refractory Multiple Myeloma (RRMM). This underscores the urgent need for innovative therapeutic approaches, particularly for high-risk RRMM patients.
Significance of BCMA
BCMA, a member of the tumor necrosis factor superfamily, is predominantly expressed in malignant plasma cells, making it an ideal target antigen for MM therapies. Various BCMA-targeted therapies, including CAR T-cell therapy, antibody-drug conjugates (ADCs), and bispecific T-cell engagers (BiTEs), have demonstrated clinical efficacy. However, achieving durable responses with these immunotherapies remains a significant challenge.
Methodology
The research team aimed to develop a novel BCMA-targeted theranostic radiopharmaceutical, focusing on its diagnostic and therapeutic potential in preclinical models of MM. The study commenced with the humanization of a rabbit monoclonal antibody (RamAb) to create the humanized BCMA-specific monoclonal antibody, BCMAh230430. The purity of the antibody was validated through SDS-PAGE (Figure 1A), and its affinity was confirmed via ELISA before coupling with DFO and labeling with 89Zr to prepare the PET probe [89Zr]Zr-DFO-BCMAh230430 (Figure 1B and 1C)
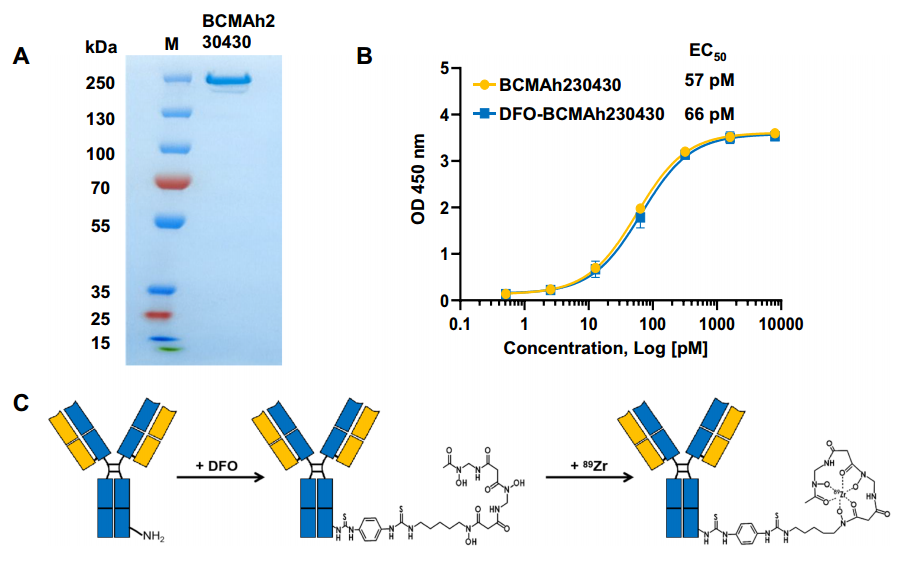
(Cited from European Journal of Nuclear Medicine and Molecular Imaging)
Figure 1. A: SDS-PAGE validation of the BCMAh230430 antibody; B: ELISA assessment of the affinity of BCMAh230430 and DFO-BCMAh230430 antibodies; C: Conjugation format of the BCMAh230430 antibody with DFO and the labeling format with [89Zr].
In Vitro and In Vivo Evaluation
Following probe preparation, the authors conducted a series of experiments, including Western Blot and flow cytometry, to screen four tumor cell lines for BCMA expression. They successfully identified MM.1S as a high-expressing cell line and KYSE520 as a low-expressing cell line (Figure 2A and 2B). Subsequent cell uptake assays further validated the targeted specificity of [89Zr]Zr-DFO-BCMAh230430 (Figure 2C).
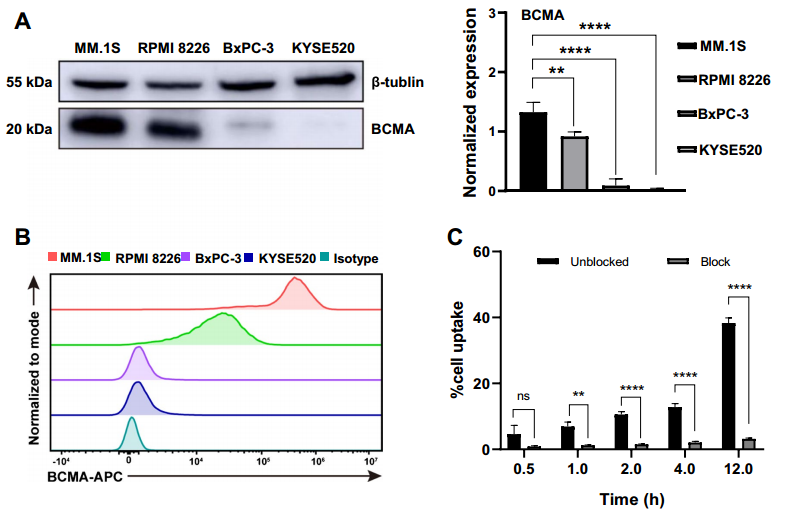
(Cited from European Journal of Nuclear Medicine and Molecular Imaging)
Figure 2. A: Western blot analysis of BCMA expression and binding capacity of BCMAh230430 antibody with tumor cells; B: Flow cytometry detection of differential BCMA expression across four cell lines; C: Uptake studies of [89Zr]Zr-DFO-BCMAh230430 in the MM.1S cell line and blocking experiments in the presence of a 100-fold excess of cold DFO-BCMAh230430 (n=3).
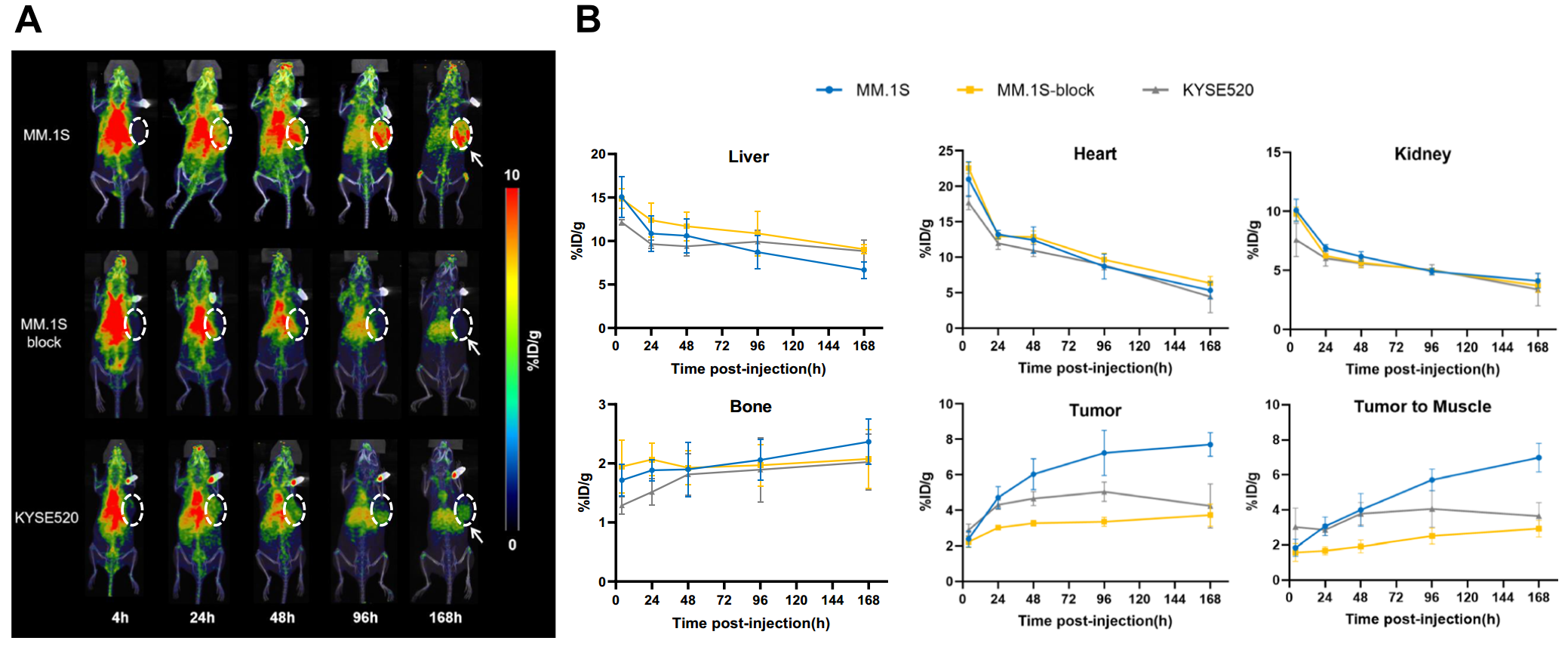
Animal models were then established using MM.1S and KYSE520 cells, with tumor growth monitored via PET imaging. Results indicated significant tumor uptake of the probe, particularly in MM.1S tumors, demonstrating the potential for non-invasive monitoring of BCMA status in MM (Figure 3A). The analysis revealed that MM.1S tumors showed nearly double the uptake compared to KYSE520 tumors (Figure 3B).
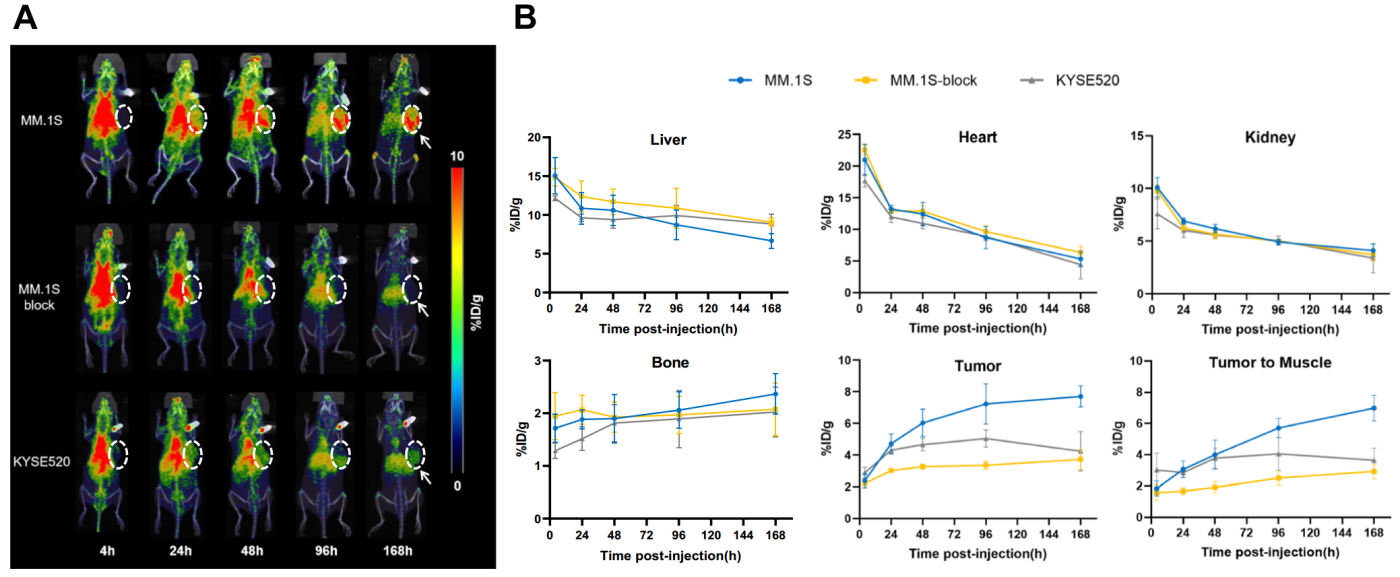
(Cited from European Journal of Nuclear Medicine and Molecular Imaging)
Figure 3. A: PET imaging of MM.1S and KYSE520 tumor models from 4 to 168 hours post-injection. B: Analysis of the region of interest (ROI) for [89Zr]Zr-DFO-BCMAh230430 immuno-PET imaging data in the MM.1S and KYSE520 groups (n=4).
To corroborate PET imaging findings, ex vivo biodistribution studies were performed, confirming a strong correlation between imaging and actual radiotracer distribution in tumor-bearing mice (Figure 4).
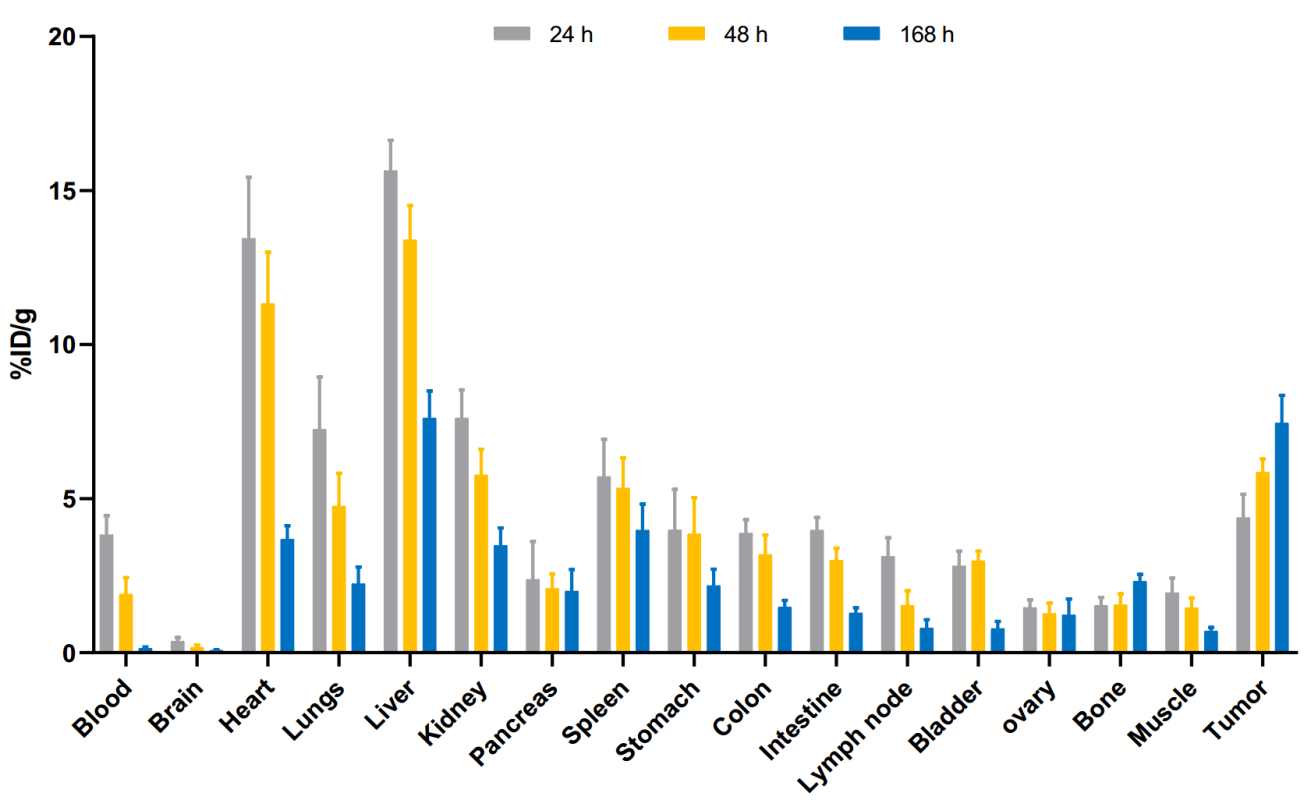
(Cited from European Journal of Nuclear Medicine and Molecular Imaging)
Figure 4. Biodistribution of [89Zr]Zr-DFO-BCMAh230430 in human MM.1S subcutaneous xenografts at 24, 48, and 168 hours post administration of the radiotracer (n=4).
Immune Histochemistry
To assess BCMA expression within tumors, the research team utilized immunohistochemistry on tumor samples fixed in formalin. Results showed a higher BCMA expression in MM.1S tumor tissues compared to KYSE520, further validating the specificity of the [89Zr]Zr-DFO-BCMAh230430 probe (Figure 5).
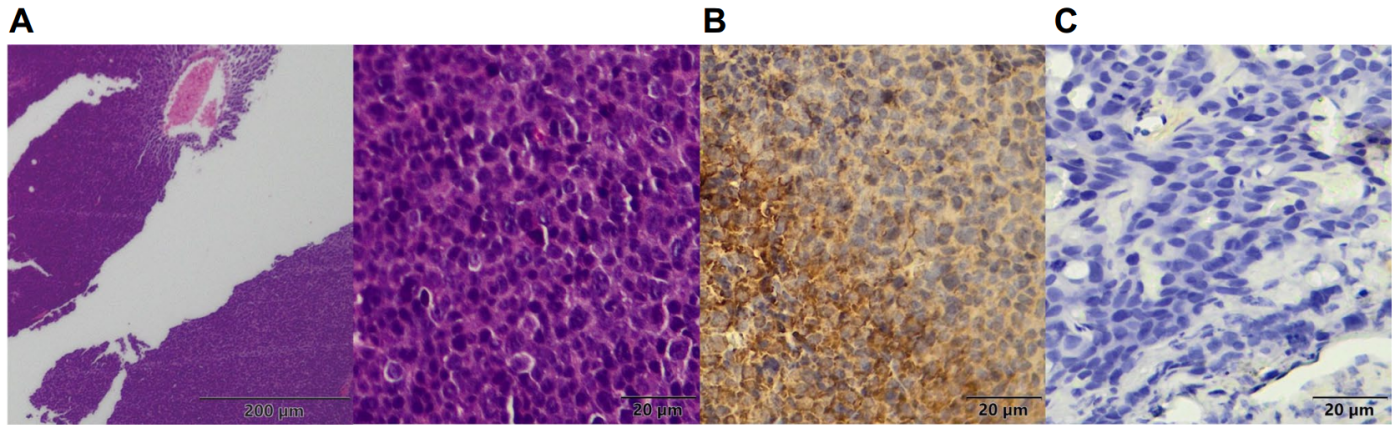
(Cited from European Journal of Nuclear Medicine and Molecular Imaging)
Figure 5. A: H&E staining of MM.1S tumor tissues; B: Immunohistochemical staining of MM.1S tumor tissues; C: Immunohistochemical staining of KYSE520 tumor tissues.
Conclusion
This study successfully validated the important role of the BCMA-targeted immuno-PET probe [89Zr]Zr-DFO-BCMAh230430 in non-invasive monitoring of BCMA status in MM tumors. The findings reveal promising tumor uptake and specific binding affinity, proposing a novel radiotherapy diagnostic approach based on BCMA antibodies that holds the potential for effective and precise diagnosis and treatment of MM.
Acknowledgments
The monoclonal antibody utilized in this study was developed by DIMA Biotechnology, a company committed to advancing antibody-based therapies for tumor-related drug targets. DIMA Biotech offers a comprehensive range of services for early-stage antibody drug discovery, accelerating the drug development process. With a focus on over 500 drug targets, DIMA Biotech has developed an extensive portfolio of in-stock antibody molecules and established a pre-validated B-cell seed library to support ongoing research efforts.