Tumor-Associated Calcium Signal Transducer 2 (TROP2), also known as TACSTD2 and EGP-1, is a surface antigen found in numerous solid tumors. It has become a pivotal target for pharmaceutical giants spurring a race in drug development in the world. Currently, TROP2-targeting biopharmaceuticals are predominantly concentrated in the Antibody-Drug Conjugate (ADC). Based on incomplete statistics, there are 11 TROP2-targeting ADC drugs in clinical development. Among these, two are in Phase III trials, seven in Phase II, and two in Phase I. Before delving into the research progress of TROP2-ADC drugs, let’s first explore the structural characteristics and functions of TROP2.
1. Structural Characteristics and Functions of TROP2
TROP2 is a cancer-associated antigen, characterized by a single-pass transmembrane surface glycoprotein with a molecular size of 36 kDa. The TROP2 protein comprises four distinct segments, including 323 amino acids: 1-26 amino acids as a hydrophobic signal peptide, 27-274 amino acids as the extracellular domain, 275-297 amino acids as the transmembrane domain, and 298-323 amino acids as the cytoplasmic tail. Furthermore, TROP2 possesses four potential N-linked glycosylation sites located within the extracellular domain, specifically at amino acid positions 33, 120, 168, and 208. The cytoplasmic tail of TROP2 exhibits a conserved structural motif crucial for TROP2-dependent signal transduction. Additionally, it contains a conserved phosphorylation site at 303 serine residues. Mutation at this site leads to the loss of TROP2’s ability to stimulate tumor growth.
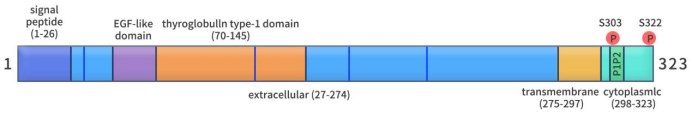
Figure 1. The structure of TROP2 [1]
TROP2 is primarily overexpressed in various human epithelial cancers, such as breast cancer, lung cancer, gastric cancer, colorectal cancer, pancreatic cancer, prostate cancer, and more. Its expression is significantly lower in normal tissues. Furthermore, the degree of TROP2 expression correlates with disease malignancy. Overexpression of TROP2 promotes tumor cell growth, proliferation, and metastasis by modulating calcium ion signaling pathways, cell cycle protein expression, and reducing fibronectin adhesion. This differential expression is what makes TROP2 an emerging target for researchers in developing ADCs.
2. Research Progress of TROP2-ADC Drugs
2.1 Phase III Clinical Trials of TROP2-ADC Drugs
SKB264 (MK-2870), developed by Sichuan Kelun Pharmaceutical Co., Ltd., is a TROP2-ADC currently in Phase III clinical trials. It is indicated for the treatment of breast cancer, non-small cell lung cancer, and triple-negative breast cancer. During the recent European Society for Medical Oncology (ESMO) congress held in October, SKB264 was introduced by Kelun Biological and MSD for the treatment of hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer (mBC). Results from Phase I/II clinical trials confirmed the controlled safety and effective anti-tumor activity of SKB264 in HR+/HER2- metastatic breast cancer patients.
Dato-DXd (Datopotamab Deruxtecan) is developed by Daiichi Sankyo Company using their proprietary DXd ADC technology, and it is a TROP2-ADC currently in Phase III clinical development. Dato-DXd is intended for the treatment of non-small cell lung cancer, triple-negative breast cancer, and breast cancer. In conjunction with SKB264, Daiichi Sankyo and AstraZeneca recently disclosed the mid-term data of the first Phase III TROPION-Lung01 study for the treatment of lung cancer during the same ESMO congress. When compared to docetaxel, Dato-DXd demonstrated a significant improvement in median progression-free survival (4.4 months vs. 3.7 months). TROPION-Lung01 marked the first positive Phase III clinical study of an ADC drug for advanced non-small cell lung cancer patients in comparison to docetaxel.
2.2 Phase II Clinical Trials of TROP2-ADC Drugs
Anti TROP2 antibody drug conjugate ADC (Fudan University), developed by Fudan University, is currently in Phase II clinical trials, targeting triple-negative breast cancer. In March 2023, Fudan University initiated Phase II clinical trials in mainland China for the treatment of triple-negative breast cancer. Recruitment for this trial has not yet begun.
STI-3258 (ESG-401), jointly developed by Shanghai Celsion BioMedTech and DongYao Pharmaceuticals, is another TROP2-ADC currently in Phase II clinical development. This compound employs a recombinant humanized anti-Trop2 monoclonal antibody linked to SN38 via a stable cleavable linker. It is intended for the treatment of bladder cancer, lung cancer, colorectal cancer, ovarian cancer, breast cancer, gastric cancer, and solid tumors. In June 2023, Celsion BioMedTech presented preliminary data from the first human study of ESG401 at the 2023 American Association for Cancer Research (AACR) Annual Meeting. Among 33 assessable patients, 12 achieved partial responses (PR), and 4 achieved disease stability (SD) for more than 24 weeks. In 11 effective doses for triple-negative breast cancer (TNBC) patients, the objective response rate (ORR) was 36% (4/11), and the disease control rate (DCR) was 64% (7/11). For 13 effective doses of HR+/HER2- breast cancer patients, ORR was 62% (8/13), and DCR was 77% (10/13).
SHRA1921, jointly developed by Shanghai Hutchison Pharmaceuticals and Suzhou Sundia Bio-Med Pharma, is a TROP2-ADC that employs a cleavable linker to connect a humanized anti-TROP2 IgG1 antibody with SHR9265. It is currently in Phase II clinical development for the treatment of salivary gland cancer and solid tumors. Preliminary preclinical data presented by Hutchison Pharmaceuticals at the 2023 AACR Meeting demonstrated that SHR-A1921 offers an extended half-life, higher stability, and enhanced safety. Convincing efficacy and good safety profiles were also observed in more than 50 subjects in a Phase I clinical trial in China, up to October 21, 2022. In assessable patients, ORR was 33.3%, and DCR was 80.0%.
FDA-018-ADC, developed by Shanghai WuXi Zhangjiang Biopharmaceuticals, is a next-generation ADC drug based on the BB05 linker-drug platform. It conjugates a TROP2 antibody with a topoisomerase I inhibitor. This TROP2-ADC is currently in Phase II clinical development for the treatment of breast cancer and triple-negative breast cancer.
BL-M02D1, jointly developed by Chengdu Boiodtech Biopharmaceuticals and Sichuan Boiodtech, is another TROP2-ADC in Phase II clinical development, employing a cleavable linker to connect a novel TROP2 antibody with Ed-04, a derivative of auristatin. It is indicated for the treatment of non-small cell lung cancer and solid tumors.
MHB-036C is developed by Minghui Medical Technology using their proprietary next-generation topoisomerase inhibitor ADC technology platform. It is a TROP2-ADC. It is currently in Phase II clinical development for the treatment of solid tumors.
9MW2921, developed by Mycenax Biotech, is an antibody-drug conjugate (ADC) and a TOP1 inhibitor. This TROP2-ADC is in Phase II clinical development for the treatment of solid tumors.
LCB84, developed by LegoChem Biosciences, is another ADC that uses their proprietary site-specific conjugation technology, ConjuAll, to link a humanized IgG1 TROP2 antibody with monomethyl auristatin E (MMAE). It is currently in Phase II clinical development for the treatment of solid tumors.
2.3 Phase I Clinical Trials of TROP2-ADC Drugs
DAC-002 (JS-108), developed by Hangzhou Doxy Biotech, is a TROP2-ADC that utilizes an intelligent linker to connect an anti-TROP2 antibody with a Tubulysin B-like molecule. This TROP2-ADC is currently in Phase I clinical development for the treatment of small cell lung cancer.
BAT8008, developed by Bio-Thera Solutions, is a TROP2-ADC in Phase I clinical development. It employs a self-developed cleavable linker to connect a recombinant humanized anti-TROP2 antibody with a toxic small molecule, a topoisomerase I inhibitor. It is intended for the treatment of solid tumors.
3. DIMA’s Contribution to TROP2-Related Drug Development
DIMA BIOTECH is a biotechnology company focusing on preclinical research products and services for druggable targets. The company boasts a robust platform for multi-transmembrane protein preparation, a unique lead antibody sequence library, an innovative antibody humanization platform, and a mature CAR T in vitro/in vivo functional analysis platform. These tools facilitate pharmaceutical companies in surmounting the hurdles of establishing a monoclonal antibody platform and lead molecule screening, allowing them more time to concentrate on the biological mechanisms and druggability research of targets, thereby accelerating pipeline development.
DIMA provides a complete range of TROP2 target products, including active proteins, flow cytometry-validated rabbit monoclonal antibodies, and affinity maturation services to support CD47 antibody drug research. Additionally, they have developed a B-cell seed library for TROP2 antibodies, enabling rapid lead molecule selection within as little as 20 days to significantly reduce your development timeline. Collaboration inquiries are welcome.
DIMA Biotech has established a comprehensive set of preclinical pharmacology evaluation platforms for ADC targets. For collaborative inquiries, please contact: info@dimabio.com
- Recombinant protein
Human Trop2 Protein, mFc-His Tag (PME100501)
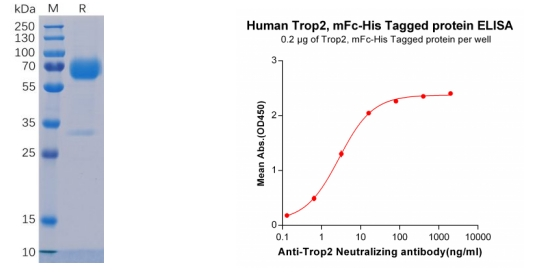
Figure 2. Validation data of purified Human TROP2 Protein, mFc-His Tag (PME100501) on SDS-PAGE (left); TROP2 protein (PME100501) can bind Anti-Trop2 Neutralizing antibody (BME100023) in a linear range of 0.13-16.0 ng/ml (right).
- Monoclonal antibody
Anti-Trop2 antibody(DM76); Rabbit mAb (DME100076)
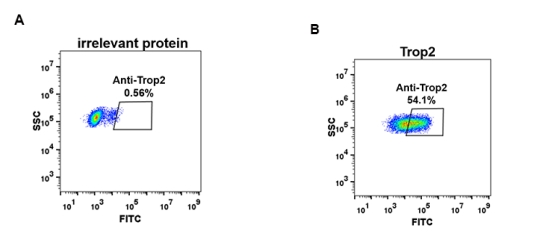
Figure 3. Expi 293 cell line transfected with irrelevant protein (A) and human Trop2 (B) were surface stained with Rabbit anti-Trop2 monoclonal antibody 1μg/ml (clone: DM76) followed by Alexa 488-conjugated anti-rabbit IgG secondary antibody.
- All products related TROP2
| Product No. | Product Name | |
| Protein | PME100766 | Human Trop2 Protein, His Tag |
| PME100501 | Human Trop2 Protein, mFc-His Tag | |
| PME-M100013 | Mouse Trop2 Protein, hFc Tag | |
| Antibody | DME100076 | Anti-Trop2 antibody(DM76); Rabbit mAb |
| DME100075 | Anti-Trop2 antibody(DM75); Rabbit mAb | |
| DME100074 | Anti-Trop2 antibody(DM74); Rabbit mAb | |
| DME100075B | Biotinylated Anti-Trop2 antibody(DM75); Rabbit mAb | |
| DME100076B | Biotinylated Anti-Trop2 antibody(DM76); Rabbit mAb | |
| DME100074B | Biotinylated Anti-Trop2 antibody(DM74); Rabbit mAb | |
| Reference antibody | BME100023 | Anti-Trop2 (sacituzumab biosimilar) mAb |
Reference:
[1] Lenart S , Lenart P , Smarda J , et al. Trop2: Jack of All Trades, Master of None[J]. Cancers, 2020.