The 116th AACR Annual Meeting will be held from April 25 to 30, 2025, at the McCormick Place Convention Center in Chicago, USA. Among the 6,470 abstracts released for the 2025 AACR, there are 340 related to antibody-drug conjugates (ADCs), covering various types such as single-target ADCs, dual-target ADCs, and dual-payload ADCs. These involve numerous popular targets, including CDH17 (12 candidates), DLL3 (10 candidates), and TROP2 (54 candidates). In this article, we review the CDH17 ADC drug development pipeline and the key players in the field.
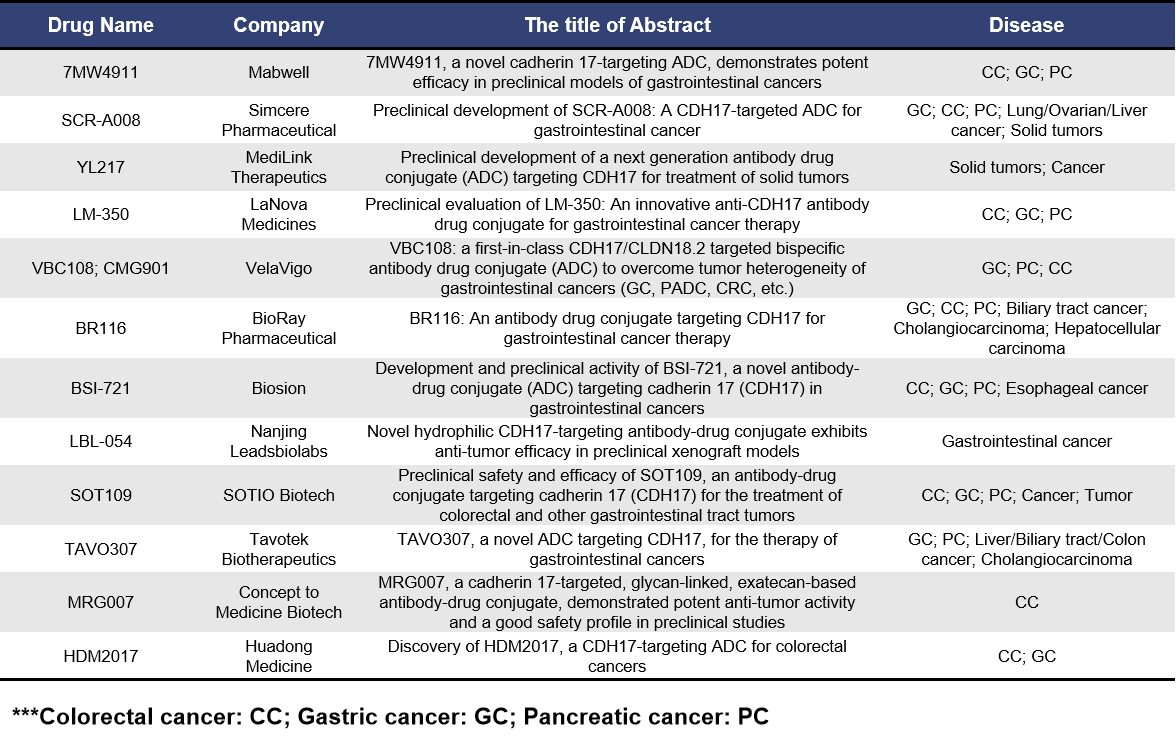
Currently, most of the companies developing CDH17-targeting ADCs are based in China. So, why are Chinese players rushing into this field? First and foremost, it’s because the target itself is highly promising. CDH17 is primarily expressed in intestinal epithelial cells and is highly expressed in gastrointestinal tumor tissues, while its expression in normal tissues is minimal. The last target with similar specificity to CDH17 was CLDN18.2. Secondly, there is a clear competitive advantage. At present, the majority of developers are Chinese companies, with only a few overseas players such as BioRay Pharmaceutical and SOTIO Biotech, both of which are still in early clinical stages. Therefore, once this target is clinically validated, there is significant potential for these drugs to expand into global markets.
- Mabwell-7MW4911
7MW4911 is an ADC drug developed by Mabwell targeting CDH17. It conjugates Mab0727, a monoclonal antibody specific to CDH17, with the novel camptothecin analogue MF-6 via a cleavable linker. This ADC utilizes a site-specific interchain disulfide bonding technology, with a drug-to-antibody ratio (DAR) of 4. At the 2025 AACR conference, Mabwell presented preclinical data on the efficacy of 7MW4911 in gastrointestinal tumors. The preclinical data indicate that 7MW4911 demonstrates exceptional efficacy, including potent activity against multidrug-resistant cancers and favorable safety profiles. The drug is currently in the preclinical stage of development globally.

- Simcere Pharmaceutical-SCR-A008
SCR-A008, developed by Simcere Pharmaceutical, is an ADC consisting of a humanized anti-CDH17 monoclonal antibody conjugated to a novel topoisomerase I inhibitor (CPT116) via a hydrophilic cleavable linker, with a drug-to-antibody ratio (DAR) of 8. The preclinical data will present at the 2025 AACR meeting suggest that SCR-A008 is a potential best-in-class therapeutic for CDH17-positive solid tumors. Like 7MW4911, SCR-A008 is also in the preclinical stage of development as a CDH17-targeting ADC.

- MediLink Therapeutics-YL217
YL217 is a novel CDH17-targeting ADC drug developed by MediLink Therapeutics, built on the company’s next-generation tumor microenvironment-activatable novel toxin-linker platform technology (TMALIN®). This platform enables the release of the payload within tumor cells and the tumor microenvironment. YL217 received FDA’s implied approval for its Investigational New Drug (IND) application in February 2025 and is poised to initiate Phase I clinical trials. At this year’s AACR conference, MediLink Therapeutics will present non-clinical data on the efficacy, safety, and pharmacokinetic characteristics of YL217. The preclinical data suggests that YL217 holds promise for further development in the treatment of CDH17-positive cancer patients.

- LaNova Medicines-LM-350
LM-350 is an ADC composed of a humanized monoclonal antibody (LM-150) conjugated to a next-generation topoisomerase I inhibitor LDX2 via a cleavable linker, with a drug-to-antibody ratio (DAR) of 8. At this year’s AACR meeting, LaNova Medicines is going to present preclinical data on LM-350, which demonstrates potent anti-tumor activity both in vitro and in xenograft models of colorectal cancer (CRC) and gastric cancer (GC). These results suggest that LM-350 has strong potential as an effective therapeutic for these types of cancers.

- VelaVigo-VBC108
VBC108 is a bispecific ADC targeting both CDH17 and CLDN18.2, designed to deliver a TOPO1 inhibitor payload that simultaneously targets these two antigens. Its unique design maximizes efficacy while minimizing safety risks. The dual-target synergy approach aims to overcome treatment challenges in gastrointestinal cancers. As a potential first-in-class (FIC) therapy, VBC108 has core advantages that facilitate rapid clinical translation. At this year’s AACR conference, VelaVigo will present preclinical data on VBC108, providing strong evidence for its potential as a leading ADC for the treatment of gastrointestinal cancers and supporting its advancement into clinical trials.

- BioRay Pharmaceutical-BR116
BR116 is an ADC composed of a highly specific humanized monoclonal antibody and an innovative topoisomerase I inhibitor, conjugated using BioRay’s unique CysX™ platform with a hydrophilic linker for stable conjugation, resulting in a drug-to-antibody ratio (DAR) of 4. At this year’s AACR conference, BioRay Pharmaceutical is to present preclinical data on BR116. The data shows that BR116 has good safety profiles in cynomolgus monkeys, indicating its potential as a therapeutic for gastrointestinal cancers. BR116 is planned for an IND application submission in 2025.

- Biosion-BSI-721
BSI-721 is an ADC composed of a fully humanized anti-CDH17 monoclonal antibody conjugated to monomethyl auristatin E (MMAE) via a cleavable peptide linker. At this year’s AACR meeting, Biosion will present preclinical activity data for BSI-721. The data shows that in a high CDH17-expressing gastric cancer model (SNU-5), BSI-721 treatment induced significant tumor shrinkage (achieving partial remission) and inhibited tumor growth (TGI). Significant TGI effects were also observed in a pancreatic cancer model (AsPC1) with low CDH17 expression. These findings highlight the potential of BSI-721 as an effective targeted therapy for gastrointestinal malignancies with varying levels of CDH17 expression. Additionally, pharmacokinetic, toxicity, and IND submission studies for BSI-721 are currently underway.
- Nanjing Leadsbiolabs-LBL-054
LBL-054 is a novel CDH17-targeting ADC developed by Leadsbiolabs using its proprietary Linker-payload technology platform. LBL-054 consists of a high-specificity CDH17 IgG1 antibody conjugated to a small molecule topoisomerase inhibitor payload via a self-developed linker, with a drug-to-antibody ratio (DAR) of 6. At this year’s AACR conference, Leadsbiolabs is to present preclinical data on LBL-054, showing that the ADC exhibits high stability in plasma, as well as strong efficacy and favorable safety profiles in in vivo models.

- SOTIO Biotech-SOT109
SOT109 is an ADC composed of a fully human anti-CDH17 monoclonal antibody conjugated to the topoisomerase I inhibitor exatecan via a hydrophobic linker. SOTIO will present preclinical data on SOT109 at the 2025 AACR conference, showing that CDH17 is highly expressed in over 90% of colorectal cancer (CRC) and other gastrointestinal cancers, making it a highly promising target. The company expects to submit an IND application for SOT109 in the second quarter of 2026.

- Tavotek Biotherapeutics-TAVO307
TAVO307 is an ADC composed of an anti-CDH17 antibody conjugated to the mitotic toxin MMAE/MMAF via a lysosome-cleavable dipeptide linker. At this year’s AACR conference, Tuochuang Biotech is going to show preclinical data on TAVO307. The data shows that in cytotoxicity assays, as well as in CDX and PDX tumor models, TAVO307 exhibited potent cytotoxic effects against multiple tumor cell lines. These results highlight the strong therapeutic potential of TAVO307 in targeting CDH17-positive tumors.

- Concept to Medicine Biotech-MRG007
MRG007 is a CDH17-targeting ADC composed of a novel humanized IgG1 antibody conjugated to the cytotoxic agent exatecan using a site-specific linkage via Synaffix’s GlycoConnect™ technology. At this year’s AACR conference, Concept to Medicine Biotech is going to present preclinical data on MRG007, which demonstrated strong anti-tumor activity and favorable safety profiles in preclinical studies. These findings underscore the potential of MRG007 as an effective therapeutic for CDH17-positive tumors.

- Huadong Medicine-HDM2017
HDM2017 is an ADC targeting CDH17, composed of a fully human monoclonal antibody specific to CDH17 conjugated to a CPT derivative payload. At this year’s AACR conference, Huadong Medicine will present preclinical data on HDM2017, showing strong in vitro and in vivo anti-tumor cytotoxicity in various gastrointestinal cancer models. The preclinical activity and safety data suggest that HDM2017 is a promising candidate for further clinical development, particularly through biomarker-based strategies.

DIMA’s ready-to-use lead molecules assist in the development of CDH17 ADC drugs
DIMA has developed over 40 lead antibody molecules targeting CDH17 using its self-developed ADC lead antibody discovery platform. Among them, 38 have been validated for cross-reactivity with both human and monkey proteins. Through flow cytometry MFI value screening, 20 clones with excellent binding affinity were selected. For some of the top-performing molecules, Dima also conducted endocytosis activity tests, cytotoxicity assays, SPR single-point detection, and epitope analysis. You can immediately access the antibody sequences and functional data validation packages, allowing you to focus on clinical progression and greatly saving time and costs. In addition, DIMA has developed a series of high-quality recombinant proteins, recombinant antibodies, and cell line products targeting the CDH17 antigen.

