In recent years, Antibody-Drug Conjugates (ADCs) have gained widespread attention as a key strategy in targeted cancer therapies. The effectiveness of ADCs relies on the specific targeting of the antibody, efficient internalization, and the release of the payload toxin. Therefore, evaluating both the internalization efficiency and cytotoxicity introduced by antibody-payload complex is crucial in ADC development. To address this need, we are excited to introduce an innovative reagent — the payload-conjugated IgG labeling reagent, designed to facilitate ADC antibody screening and accelerate drug development.
1. Innovative Products for Dual Evaluation
Traditional methods for detecting antibody internalization often rely on fluorescent labeling. However, these methods can’t directly measure the cytotoxic effects caused by the antibody. Our new reagent goes a step further by combining a toxin payload with an Fc-binding protein. This allows us to assess both the internalization of the antibody and the cytotoxicity it causes at the same time. This provides a simple, one-stop solution for screening ADC antibodies and mimics the actual action of ADC drugs (Figure 1).

Figure 1: Overview of the antibody internalization reagents, highlighting the pH sensitivity and payload conjugation.
The payload conjugated reagent includes an Fc-binding protein that is conjugated to a toxin payload. When this reagent binds to an antibody, it forms a complex that behaves like an ADC drug. This complex binds to specific target proteins on the surface of cells, enters the cells, and releases the toxin. By measuring cell inhibition using the CCK8 method, we can assess two key factors:
- Internalization – how effectively the antibody-payload complex enters the cells.
- Cytotoxicity – the effectiveness of the toxin in killing the cells after being internalized.
This dual evaluation system is a great tool for screening ADC antibody candidates without the need for conjugating the payload to each antibody individually. Researchers can use our reagent to label different IgG molecules following a standard protocol, mimicking the ADC process for fast initial screening.
2. How our reagent works:
- Binding: The Fc-binding protein conjugated with a payload toxin binds to the antibody.
- Targeting: The antibody-payload complex attaches to the target protein on the surface of cells.
- Internalization: The complex is internalized by the cells.
- Cytotoxicity: Once inside the cells, the payload toxin is released, leading to cell death. The cytotoxicity can be measured using the CCK8 method to track cell inhibition.
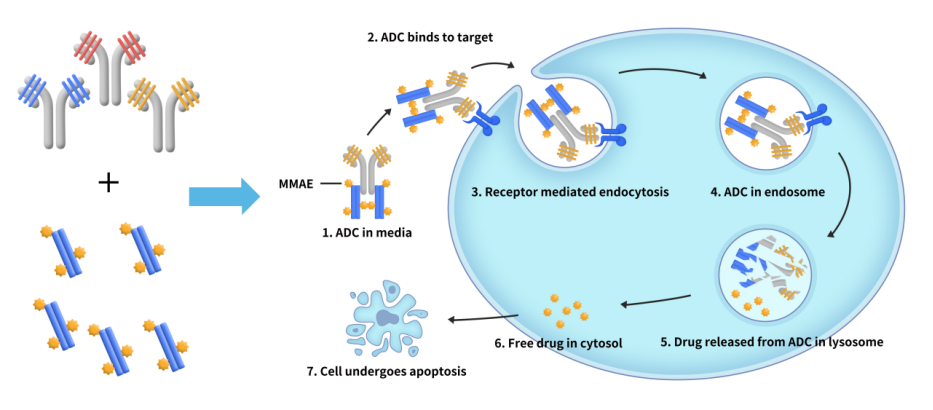
Figure 2: Step-by-step breakdown of how our antibody internalization reagent works.
This process mimics the mechanism of ADC drugs, making it easier for researchers to evaluate both internalization and cytotoxicity in a single experiment.
Currently, we offer the MMAE-conjugated IgG labeling reagent (Cat. No. AME100003). We’re also working on other conjugated toxins like Dxd, SN-38, and DM1, with more options in development. This diverse range of products provides a flexible solution for ADC screening and helps researchers find the best candidates for their therapies.
3. Compatible IgG Isotypes
Our reagent works with a variety of IgG isotypes, making it versatile for different antibody screening needs. Below is a table listing the IgG isotypes that can be screened with our AME100003 antibody internalization reagent:
| Speies | Immunoglobulin | AME100003 |
| Human | IgG(normal) | ++++ |
| IgG1 | ++++ | |
| IgG2 | ++++ | |
| IgG3 | – | |
| IgG4 | ++++ | |
| IgM | – | |
| IgA | – | |
| IgE | – | |
| IgD | – | |
| Fab | ++ | |
| κ light chains | – | |
| L light chains | – | |
| ScFv | ++ | |
| Mouse | IgG1 | + |
| IgG2a | ++++ | |
| IgG2b | +++ | |
| IgG3 | ++ | |
| Rabbit | IgG | ++++ |
4. Advantages
- Wide Compatibility: Works with most IgG isotypes from human, mouse, and rabbit, making it ideal for a variety of antibody screening needs.
- Simulates ADC Mechanism: Accurately mimics the ADC mechanism by evaluating both antibody internalization and cytotoxicity in a single step, supporting effective ADC drug development.
- Efficient & Convenient: No need for individual conjugation of toxins to each antibody, significantly reducing both experimental costs and complexity.
- Flexible Detection Methods: Compatible with fluorescence microscopy, flow cytometry, and cell viability assays (CCK-8, MTT), offering versatile tools for screening and analysis.
- Proven Stability: The reagent demonstrates high stability (Figure 3).
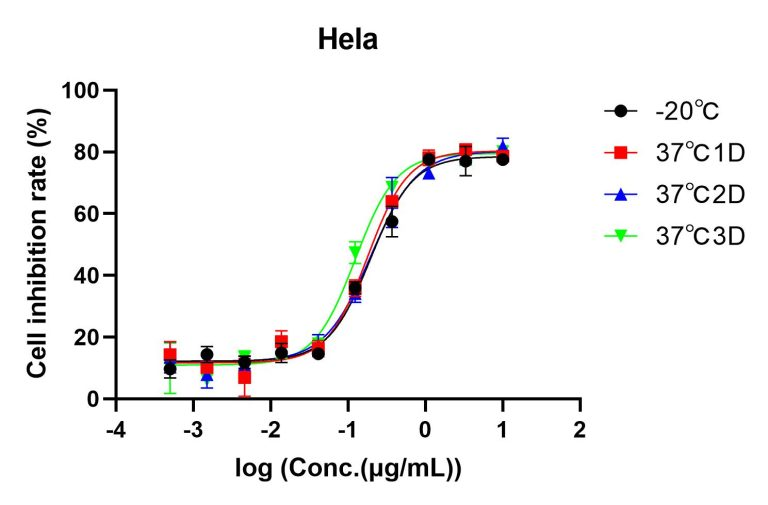
Figure 3. Accelerated stability test of AME100003. Following lyophilization, samples were stored at -20°C (black), 37°C for 1 day (red), 37°C for 2 days (blue), and 37°C for 3 days (green), separately. Upon reconstitution, the cell inhibition rate of each sample was determined using the CCK8 method. The data indicate excellent stability for all samples.
5. Case Study
To validate the effectiveness of the MMAE-conjugated IgG labeling reagent (Cat. No. AME100003) for ADC antibody screening, we performed two detailed case studies.
In this experiment, we screened 11 different monoclonal antibody clones targeting CDH17 using AME100003 and the CDH17-expressing SNU-5 cell line. The antibodies were evaluated for their internalization efficiency and cytotoxicity. The results allowed us to identify the top 3 ADC candidates (Clone 2, 3 & 6)based on the most effective cytotoxic response.
Screening CDH17 ADC Candidate Antibodies
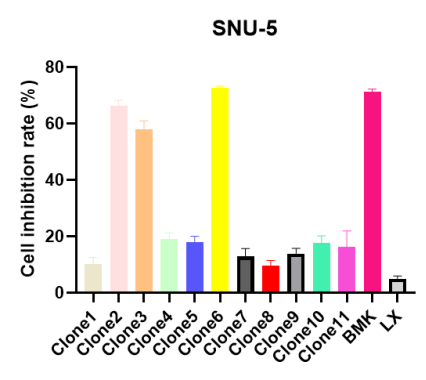
Figure 4. Evaluation of cytotoxicity of different anti-CDH17 mAbs on SNU-5 cells. Positive Control BMK: Benchmark antibody against CDH17; Negative Control LX: Benchmark antibody against GPRC5D
This case demonstrated how AME100003 can be used to quickly and effectively identify the best ADC candidates for further development.
6. Try It Now
Our reagent provides an efficient solution for ADC antibody screening, helping researchers identify candidate antibodies with strong internalization and cytotoxicity. We’re excited to offer a 50% discount on our standard product, AME100003, available for immediate trial. Additionally, if you’re interested in testing other payload-conjugated reagents (such as Dxd, SN-38, DM1, etc.), we can provide these as custom products tailored to your needs.
This is an excellent opportunity to experience our innovative reagent and accelerate your ADC drug development efforts. Offer valid until April 30, 2024.
To take advantage of this limited-time offer, simply contact our customer service team online or call our order hotline at 400-006-0995 or 18062749453. We look forward to helping you advance your ADC screening process!
