The 2024 American Society of Clinical Oncology Annual Meeting (ASCO 2024) will take place in Chicago, USA, from May 31st to June 4th. As a major event in the cancer treatment community, it will reveal many important clinical research outcomes and the latest innovations. Antibody-drug conjugates (ADCs), which cleverly combine targeted antibody therapies with powerful chemotherapy drugs, have been particularly successful in targeting and destroying cancer cells with precision and efficiency. These advancements have made ADCs a key focus in the development of new cancer treatments. Based on the abstracts shared by ASCO, we have put together an update on the progress of ADC drug targets, as highlighted in both oral and poster presentations.
1. CLDN18.2
2. HER2 and/or TROP2
3. c-Met
4. FRα
5. nectin-4
6. B7-H3
7. CD20
8. CD46
9. CD79b
10. CDH6
11. CEACAM5
12. EGFR
13. EphA5
14. HER3
15. MUC1
16. ROR1
17. SEZ6
18. TF
19. ITGB6
1. CLDN18.2
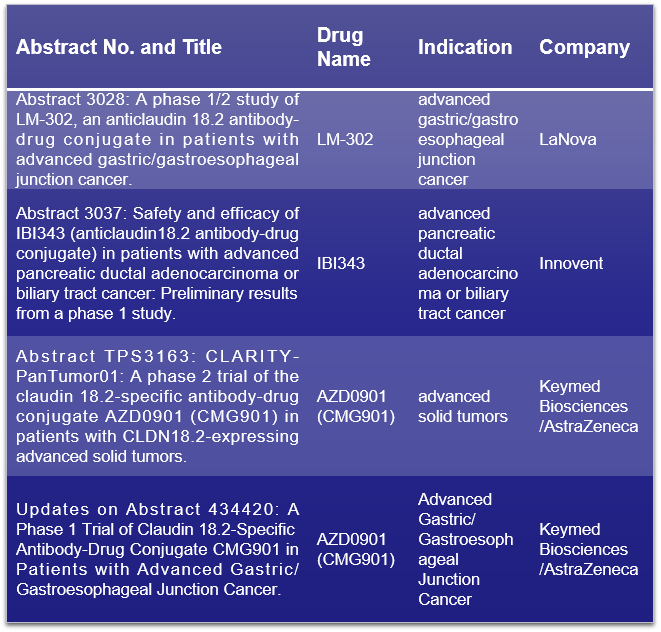
Claudin-18.2 (CLDN18.2), also known as Claudin-18 splice variant 2, is a gastric-specific tight junction protein expressed in various cancers. At the 2024 ASCO Annual Meeting, several advancements related to CLDN18.2 ADC drugs will be unveiled, shedding light on their potential impact on cancer treatment. LaNova will present the Phase I/II clinical trial results of the CLDN18.2 ADC drug LM-302 in patients with advanced gastric/esophagogastric junction cancer. Innovent Biotech will disclose the preliminary results of a Phase I study on the safety and efficacy of IBI343 in patients with advanced pancreatic ductal adenocarcinoma or biliary tract cancer. Two clinical data sets of AZD0901 (CMG901), A first-in-class CLDN18.2 ADC, co-developed by Keymed Biosciences and AstraZeneca, will be presented. One includes Phase II trial results in patients with solid tumors expressing CLDN18.2 (CLARITY-PanTumor01), and the other includes Phase I trial results in patients with advanced gastric or gastroesophageal junction cancer. Click to read more about Claudin-18.2 >>
2. HER2 and/or TROP2
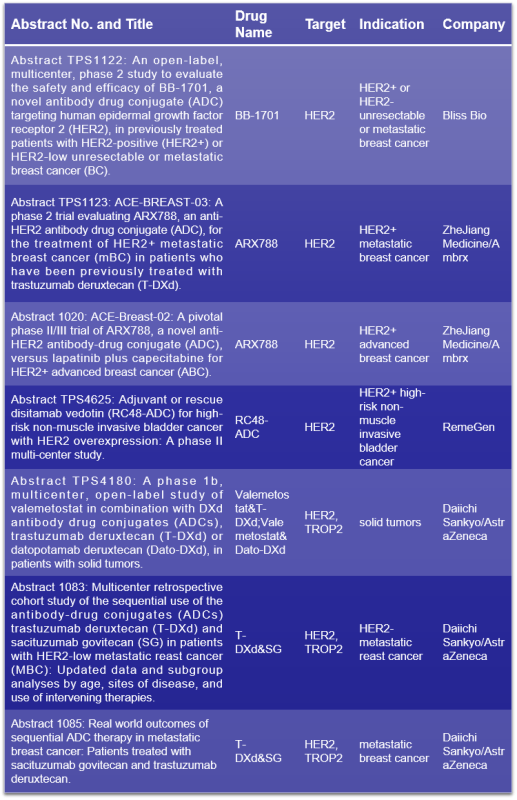
HER2 is a transmembrane receptor tyrosine kinase. Under normal circumstances, HER2 is expressed at low levels in various tissues, primarily promoting cell proliferation and inhibiting apoptosis. However, overexpression of HER2 may lead to uncontrolled cell growth and tumor formation. It is anticipated that seven studies on HER2 ADC drugs will be presented at the 2024 ASCO conference, including three comparing the efficacy of HER2 ADC drugs either in combination with or as monotherapy alongside TROP2 ADC drugs (click to know more about TROP2>>). The HER2 ADC drugs to be discussed at this conference include BB-1701, ARX788, RC48-ADC, and trastuzumab deruxtecan (T-DXd), with RC48-ADC and T-DXd already approved for marketing.
3. c-Met
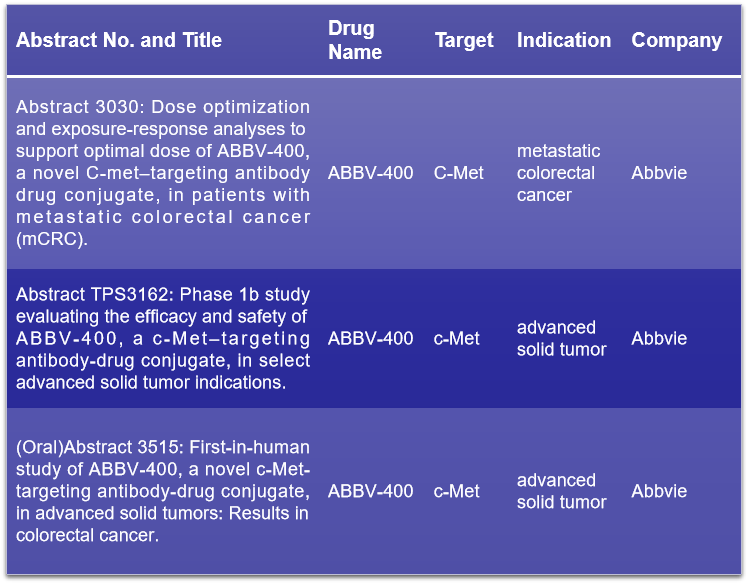
c-Met, also known as MET, serves as the receptor tyrosine kinase for hepatocyte growth factor (HGF). Studies have shown that c-Met, upon HGF binding, orchestrates a range of cellular functions, including differentiation, proliferation, movement of epithelial cells, angiogenesis, and the process of epithelial-mesenchymal transition (EMT). Disruptions in the HGF/c-Met signaling axis are implicated in the oncogenesis and progression of various cancers. During the 2024 ASCO conference, AbbVie is set to unveil three studies pertaining to the c-Met targeting ADC, ABBV-400. This includes an oral presentation detailing preliminary human trial findings in advanced colorectal cancer patients. Complementing this, two additional studies will discuss dose refinement and exposure-response assessments to ascertain the most effective dosage for metastatic colorectal cancer (mCRC) patients, alongside Phase I clinical trial outcomes that evaluate the efficacy and safety profile in advanced solid tumor cases.
4. FRα
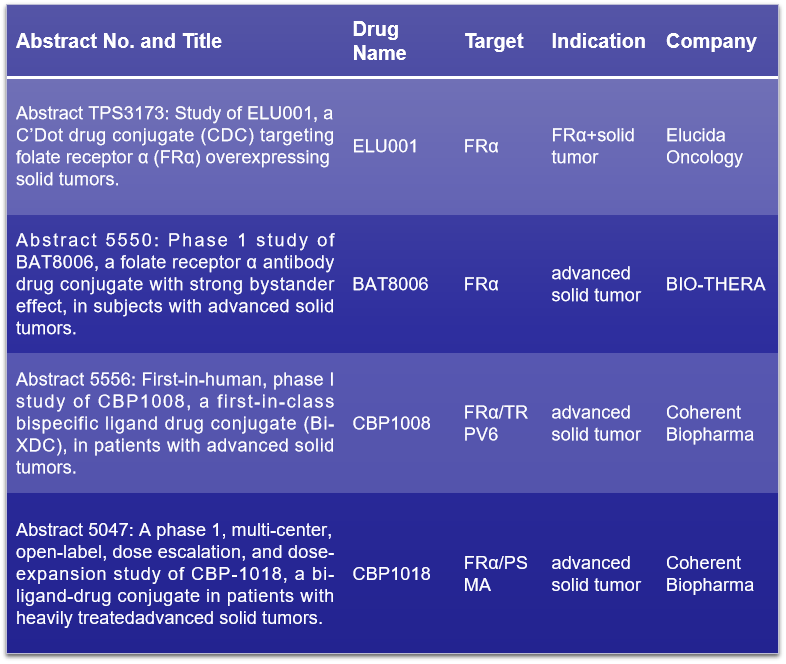
FRα (folate receptor alpha), a member of the folate receptor family, is produced from the FOLR1 gene. It has been confirmed that FRα is almost not expressed in normal cells but is widely overexpressed in solid tumors. At the 2024 ASCO conference, Elucida Oncology is set to reveal the findings of ELU001 for FRα+ solid tumors. Baiotai will unveil Phase I clinical trial outcomes of BAT8006 in advanced solid tumor patients. Additionally, Tongyi Medicine will showcase Phase I clinical trial insights for two FRα-targeting bispecific ADCs: CBP1008 and CBP1018, which are directed against FRα/TRPV6 and FRα/PSMA, respectively. Discover more about FRα and its implications in cancer therapy. Click to read more about FRα>>
5. nectin-4
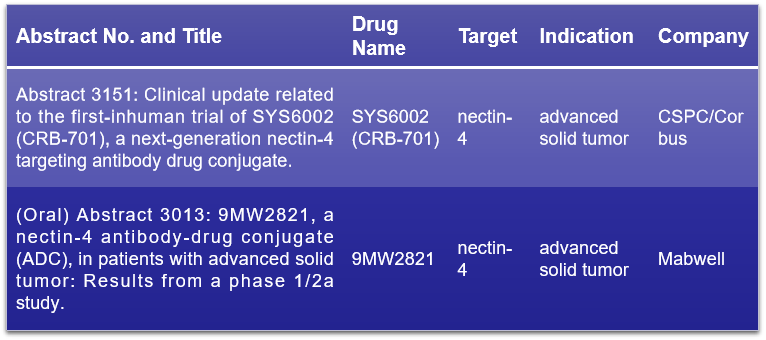
Nectin-4 belongs to the Nectin family and is a Ca2+-independent immunoglobulin superfamily cell adhesion molecule (CAM) that is overexpressed in various human malignant tumors. This abnormal expression is associated with cancer progression and poor prognosis. At the 2024 ASCO conference, it is expected that two clinical data sets on nectin-4 ADC drugs will be presented, including one oral presentation. The oral presentation will focus on 9MW2821 developed by Mabwell Biotech, while the other presentation will feature SYS6002 (CRB-701) co-developed by CSPC Group and Corbus.
6. B7-H3
B7-H3, identified as CD276, is differentially expressed in tumor versus normal tissues and has been implicated in tumor promotion, making it a compelling target for immunotherapy. Minghui Medical is slated to present the clinical Phase I/II study results of MHB088C, an ADC targeting B7-H3, at the 2024 ASCO conference. The study focuses on patients with recurrent or metastatic solid tumors and will feature in a late-breaking oral presentation. MHB088C, developed through Minghui’s SuperTopoi™ ADC platform, has shown promising efficacy and a favorable safety profile in over 150 patients across various cancer types. Click to read more about B7-H3 >>

7. CD20
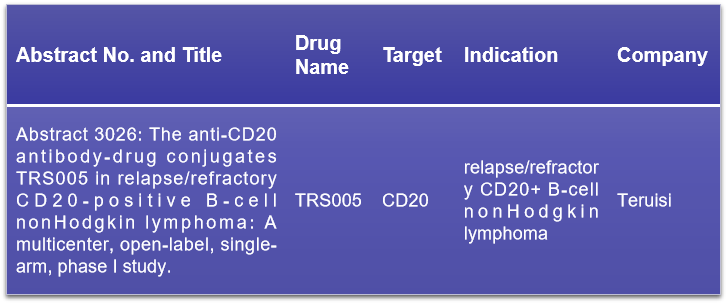
CD20, a marker of B cells, is part of the transmembrane 4 superfamily A (MS4A) protein family. It is present on the surface of both normal and about 95% of malignant B lymphocytes, yet it is not found on hematopoietic stem cells, plasma cells, or other normal tissues. At the upcoming conference, Teruisi Pharmaceutical is expected to present the Phase I clinical research data for their CD20 ADC drug, TRS005, which targets relapsed or refractory CD20-positive B-cell non-Hodgkin lymphoma. The study aims to evaluate the safety, pharmacokinetics, and preliminary efficacy of TRS00512. This research could provide valuable insights into the potential of TRS005 as a therapeutic option for patients with this type of lymphoma.
8. CD46
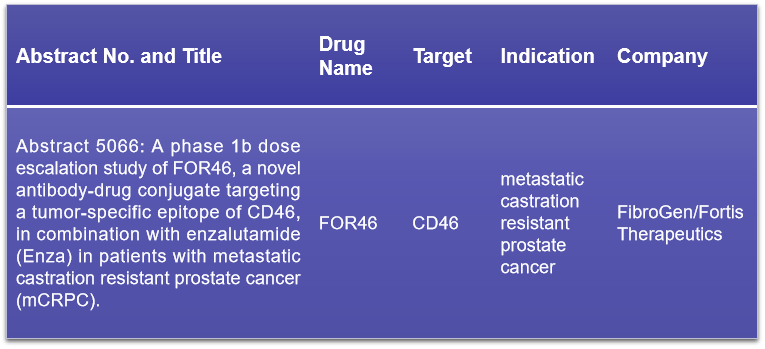
CD46, recognized as membrane cofactor protein, is a type I membrane protein integral to the complement system’s regulation. It is ubiquitously expressed on normal cells and facilitates the cleavage of C3b and C4b, playing a pivotal role in modulating both the classical and alternative complement activation pathways. The association of CD46 with diverse immune-inflammatory diseases has been well-documented. At the upcoming conference, a Phase I clinical data presentation is eagerly awaited, which will discuss FOR46, an ADC drug targeting CD46. This drug is a collaborative development by FibroGen and Fortis Therapeutics, and it has shown promise in treating metastatic castration-resistant prostate cancer (mCRPC), with clinical activity observed and an acceptable safety profile akin to other MMAE-containing ADCs. The presentation will likely provide valuable insights into the efficacy and safety of FOR46, furthering the understanding of CD46 as a therapeutic target.
9. CD79b
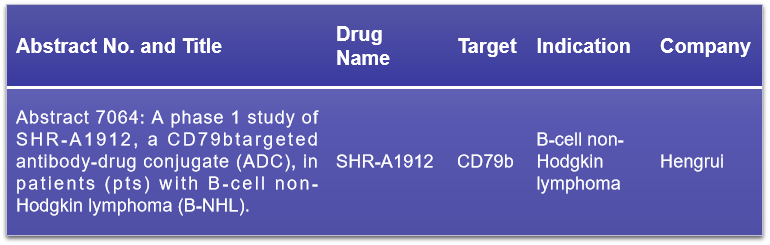
CD79b, a key signaling component of the B-cell antigen receptor (BCR), pairs with CD79a to form the BCR complex by non-covalently associating with membrane immunoglobulin (mIg). This interaction is crucial for initiating B-cell activation. CD79b’s high specificity to B-cells and its elevated expression in various B-cell lymphomas make it an effective target for therapies aimed at abnormal B-cell proliferation. At the 2024 ASCO conference, Hengrui Medicine is set to present the Phase I clinical data of SHR-A1912, an ADC targeting CD79b, which could offer new hope for patients with B-cell lymphomas. The presentation will likely provide insights into the safety, tolerability, pharmacokinetics, and preliminary efficacy of SHR-A1912, marking a significant step in the development of targeted therapies for hematological malignancies.
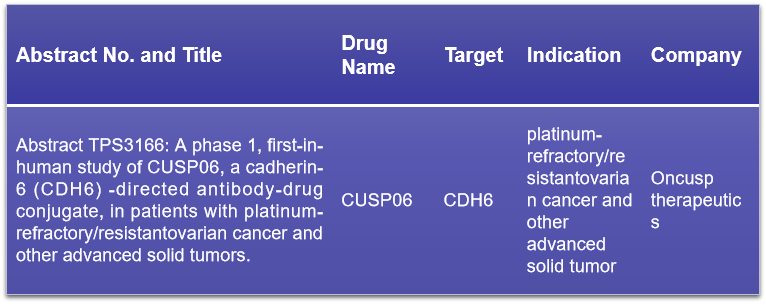
10. CDH6
CDH6, a type II classic cadherin and single-pass transmembrane protein, has been implicated in various oncogenic processes. Its overexpression is linked to tumor growth and proliferation, playing roles in cell-cell adhesion, organ development, and epithelial-mesenchymal transition (EMT). At the 2024 ASCO conference, Oncusp therapeutics is poised to present the Phase I clinical results of CUSP06, their innovative CDH6-targeting ADC drug. This presentation will likely cover the safety, tolerability, pharmacokinetics, and preliminary efficacy of CUSP06 in patients with advanced solid tumors. The anticipation for these results is high, as they could mark a significant advancement in the treatment of cancers associated with CDH6 overexpression.
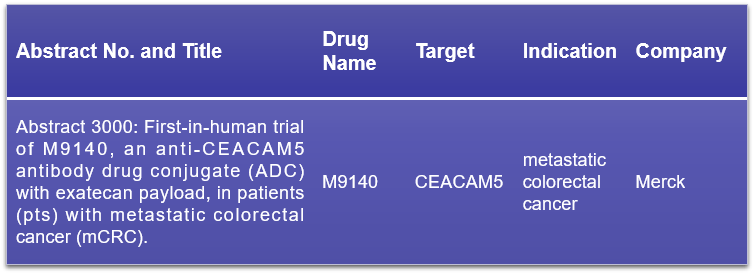
11. CEACAM5
CEACAM5, also recognized as CEA or CD66e, is a pivotal member of the carcinoembryonic antigen-related cell adhesion molecule family. Typically, CEACAM5 expression is confined to the apical surface of epithelial cells in the lungs and gastrointestinal tract, rendering it inaccessible to immune cells. However, in cancerous cells, CEACAM5 is notably overexpressed on the surface, particularly in a variety of epithelial tumors such as colorectal, gastric, pancreatic, lung adenocarcinoma, small cell lung cancer, bladder, and ovarian cancers. This conference will feature Merck’s presentation on the initial human dosing results of its CEACAM5-targeted ADC drug, M9140. The study aims to assess the safety, tolerability, pharmacokinetics, and preliminary clinical activity of M9140 in advanced solid tumors. Click to read more about CEACAM5 >>
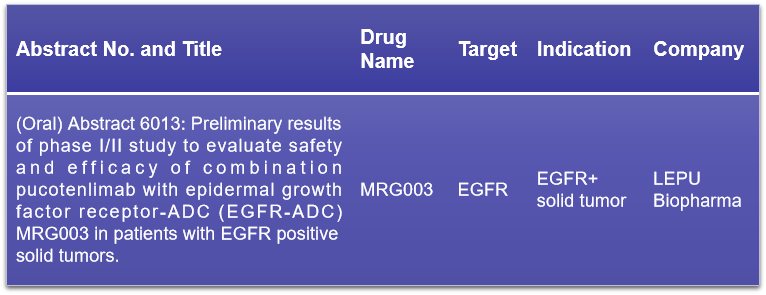
12. EGFR
EGFR, or epidermal growth factor receptor, is a transmembrane glycoprotein that belongs to the receptor tyrosine kinase ERBB family. It’s a well-established target in anti-tumor therapy. Despite the availability of numerous EGFR-targeting drugs, resistance remains a challenge, prompting the development of new therapeutics. LEPU Biopharma will present preliminary findings on the safety and efficacy of the EGFR ADC drug MRG003 in combination with pucotenlimab for patients with EGFR-positive solid tumors. The study’s results are promising, indicating that MRG003, when used with pucotenlimab, shows a manageable safety profile and potential efficacy in treating patients who have shown resistance to other treatments. This combination therapy could represent a significant advancement in the treatment of EGFR-positive tumors.
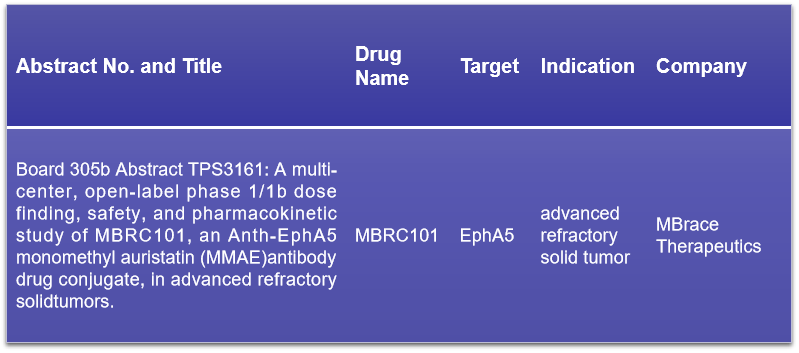
13. EphA5
EphA5, a receptor tyrosine kinase, is increasingly recognized for its presence in various cancers, including breast cancer, NSCLC, colorectal, gastric, and pancreatic cancers. MBrace Therapeutics is set to present the results of the Phase I/1b clinical trial for their EphA5-targeting ADC drug, MBRC101, at the ASCO conference. The trial aims to evaluate the safety, pharmacokinetics, and anti-tumor activity of MBRC101, which has shown promise in preclinical studies. This presentation is highly anticipated as it could provide valuable insights into the potential of MBRC101 as a novel therapeutic option for patients with EphA5-positive tumors.
14. HER3
HER3, part of the epidermal growth factor receptor (EGFR) family, commonly forms heterodimers with EGFR or HER2 to fulfill its biological role. In cancerous tissues, an overexpression pattern of EGFR and HER2 often includes HER3 as well. Historically, HER3’s minimal kinase activity led to its underestimation as a therapeutic target. However, its significant role in tumor development and resistance to drugs has recently brought HER3 into the spotlight. At the current ASCO conference, Medilink Therapeutics is set to unveil the outcomes of the inaugural human dosing trial for its HER3-targeting ADC drug, YL202, a collaborative effort with BioNTech.

15. MUC1
MUC1, also referred to as MAM6 and EMA, is a mucin protein that plays a significant role in various epithelial-derived tumors, including lung, pancreatic, prostate, ovarian, and breast cancers. Its overexpression is linked to tumor metastasis and recurrence, making it a critical target for cancer therapy. Daiichi Sankyo’s DS-3939a, a novel MUC1-targeting ADC, has shown potent antitumor activity in preclinical models and is now advancing through clinical trials. The first human dosing clinical trial results will be presented at the 2024 ASCO conference, marking a significant milestone in the development of targeted cancer therapies. Click to read more about MUC1 >>

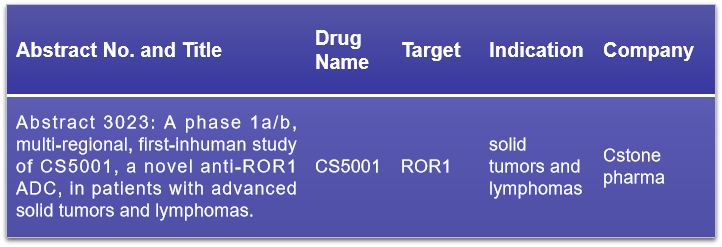
16. ROR1
ROR1, a type I transmembrane protein and part of the receptor tyrosine kinase-like orphan receptor family, is distinctively expressed in various solid tumors and hematologic malignancies but not in normal adult tissues. This unique expression pattern positions ROR1 as a promising target for broad-spectrum anti-cancer therapies. Cstone pharma is set to present the clinical trial results of CS5001, their ROR1-targeting ADC drug, at this conference. The trial results are particularly noteworthy as CS5001 incorporates a tumor-cleavable linker and a pyrrolobenzodiazepine (PBD) prodrug, which releases the PBD toxin specifically within the tumor environment, potentially reducing systemic toxicity and improving the safety profile. Click to read more about ROR1 >>
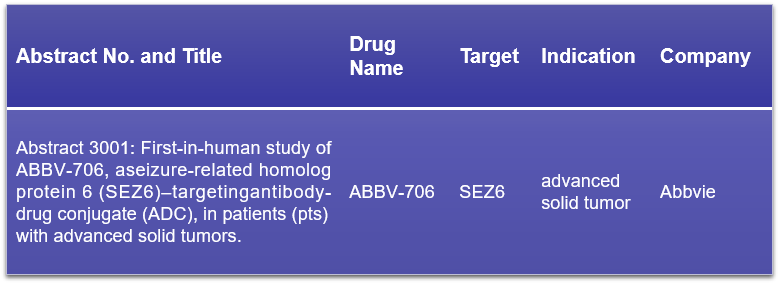
17. SEZ6
SEZ6, or seizure-related 6, is indeed a transmembrane protein found on certain neuronal lineage cells. While its expression in normal tissues is minimal, SEZ6 is significantly overexpressed in small cell lung cancer (SCLC), which has positioned it as a prime target for antibody-drug conjugates (ADCs). AbbVie’s ABBV-706, an innovative SEZ6-targeted ADC, has completed dose escalation and is currently undergoing dose expansion in a Phase 1 first-in-human clinical study. The study includes ABBV-706 monotherapy dose escalation in relapsed or refractory solid tumors, and dose optimization and expansion in SCLC and neuroendocrine cancers. The results, which are highly anticipated, will provide insights into the safety, pharmacokinetics, and efficacy of ABBV-706, potentially offering a new therapeutic avenue for patients with late-stage solid tumors.
18. TF
Tissue Factor (TF), also known as Factor III, CD142, or thromboplastin, is a transmembrane glycoprotein that plays a pivotal role in the coagulation cascade. While TF is not typically expressed in the endothelial cells of normal tissue blood vessels, it is highly expressed in a variety of tumors, including cervical, breast, prostate, colon, esophageal, and pancreatic cancers. This differential expression makes TF an attractive target for ADC (antibody-drug conjugate) therapies. Lepu biopharma’s MRG004A is one such ADC drug that targets TF, and it has been granted approval for clinical use. The drug has been through a Phase I/II clinical trial registered in the U.S. for the treatment of advanced solid tumors, with 181 patients enrolled. The results of this trial are set to be announced at the 2024 ASCO conference, which will provide valuable insights into the efficacy and safety of MRG004A as a potential new treatment option for patients with TF-positive solid tumors. The anticipation for these results is significant, as MRG004A represents a new class of targeted therapy that could potentially improve outcomes for patients with cancers that overexpress TF.

19. ITGB6
ITGB6, the β6 subunit of the integrin protein αvβ6, is a critical component in the regulation of this heterodimer’s expression. Its overexpression in various solid tumors has been associated with poor prognostic outcomes in cancer patients. Pfizer’s SGN-B6A, an ITGB6-targeted antibody-drug conjugate (ADC), has been under investigation for its efficacy and safety in treating patients with non-small cell lung cancer (NSCLC). The latest clinical trial update for SGN-B6A will be announced at the conference, which is expected to provide updated results from the ongoing phase 1 study focusing on NSCLC, head and neck squamous cell carcinoma (HNSCC), and esophageal cancer (EC). The study, SGNB6A-001, is an open-label, multicenter, dose-escalation/expansion study evaluating the safety, pharmacokinetics, and antitumor activity of SGN-B6A.


