On February 27, 2024, Viking Therapeutics announced the results of their Phase IIa clinical study for VK2735, a dual GLP-1R/GIPR agonist for obesity. The results showed a maximum weight loss of 13.1% after 13 weeks of treatment. Following this news, Viking’s stock surged by 121%. The company has received written feedback from the FDA and plans to advance this pipeline to Phase III development. VK2735 could become a direct competitor to Eli Lilly’s Mounjaro, currently the only approved dual GLP-1/GIP receptor agonist. Today, we will discuss the background of GIPR and the current competitive landscape of clinical drugs targeting this receptor.
1. GPCR Family
The Gastric Inhibitory Polypeptide (GIP) receptor (GIPR) belongs to the G Protein-Coupled Receptor (GPCR) superfamily. GPCRs are the largest protein family encoded by the human genome. Located on the cell membrane, they convert extracellular signals into crucial physiological effects. GPCRs are characterized by a structure with seven transmembrane domains, which contribute to their diverse downstream signaling pathways, making them highly attractive for drug development. The human GPCR family is categorized into four main subfamilies based on amino acid sequences: Class A (rhodopsin-like family), Class B (secretin and adhesion), Class C (glutamate), and Class F (frizzled) [1]. Class A GPCRs, consisting of 719 members, are further divided into several subtypes, including adrenergic, peptide, protein, lipid, melatonin, nucleotide, steroid, fatty acid, sensory, and orphan subtypes. Class B GPCRs are further divided into two subfamilies: secretin (B1) and adhesion (B2), with 15 and 33 members respectively. Class C comprises 22 receptors, subdivided into five subfamilies: calcium-sensing receptor (CaSR), GABA B receptors (GABA B1 and GABA B2), taste receptors (TS1R1, TS1R2, and TS1R3), 8 metabotropic glutamate receptors (mGluR1–8), and 8 orphan GPCRs. Class F includes 11 members, with 1 smoothened receptor (SMO) and 10 frizzled receptors (FZD1-10) [2].
2. GIPR Structure and Distribution
GIPR is specifically a Class B1 GPCR that interacts with glucagon-like peptides (GLP). The GIPR protein features a large extracellular N-terminal domain, seven transmembrane domains, and a short intracellular C-terminal domain. The N-terminal domain contains six highly conserved cysteine residues forming three disulfide bonds. The C-terminal intracellular domain mediates intracellular signal transduction by physically interacting with G proteins. GIPR is widely distributed in the body, expressed in the pancreas, stomach, small intestine, adipose tissue, heart, and brain, with the highest expression in pancreatic β-cells. Upon activation by GIP, GIPR binds to the heterotrimeric Gs protein (αβγ), leading to the activation of adenylate cyclase, which increases intracellular cAMP levels. Elevated cAMP activates Protein Kinase A (PKA), leading to the phosphorylation of regulatory gene transcription proteins and their subsequent translocation to the nucleus.
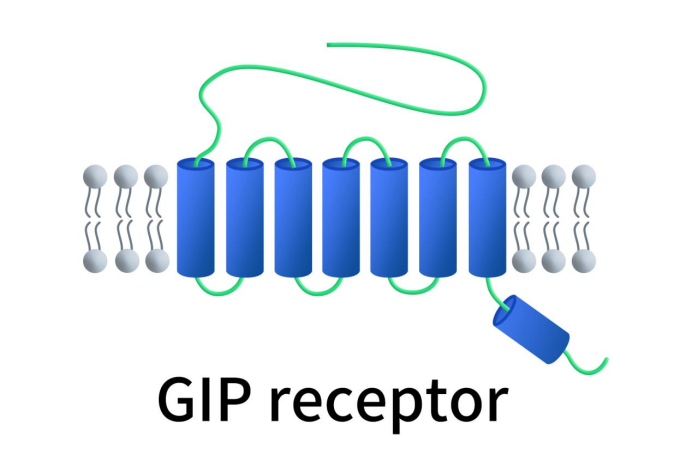
Figure 1. The structure of GIPR
3. Mechanism of the GIP/GIPR Signaling Pathway
GIP, also known as glucose-dependent insulinotropic polypeptide, is one of two gut incretin hormones, the other being glucagon-like peptide 1 (GLP-1). These hormones link nutrient intake to systemic metabolism. GIP has various effects on different systems, primarily enhancing insulin secretion from pancreatic β-cells following food intake (known as the incretin effect). Research shows that after a meal, when blood glucose levels rise, GIP binds to GIPR on the surface of β-cells in the pancreas. This interaction activates the adenylate cyclase-cyclic AMP-Protein Kinase A (AC-cAMP-PKA) pathway, leading to the opening of downstream calcium channels. The influx of calcium ions increases, stimulating the transcription of proinsulin genes and thus promoting insulin secretion.
Under fasting or hypoglycemic conditions, free fatty acids bind to Peroxisome Proliferator-Activated Receptor Alpha (PPARα). PPARα then forms a heterodimer with Retinoid X Receptor (RXR) and translocates to the nucleus, where it binds to Peroxisome Proliferator Response Elements (PPRE), stimulating GIPR transcription and increasing GIPR expression on the cell surface. However, high glucose levels inhibit PPARα transcription through Glucocorticoid Response Elements (GRE) within the PPARα promoter, leading to decreased PPARα transcription and expression. With reduced PPARα levels, it can no longer fully stimulate GIPR expression, resulting in decreased GIPR expression. This reduction in GIPR expression leads to decreased insulin secretion in response to GIP in β-cells [3].
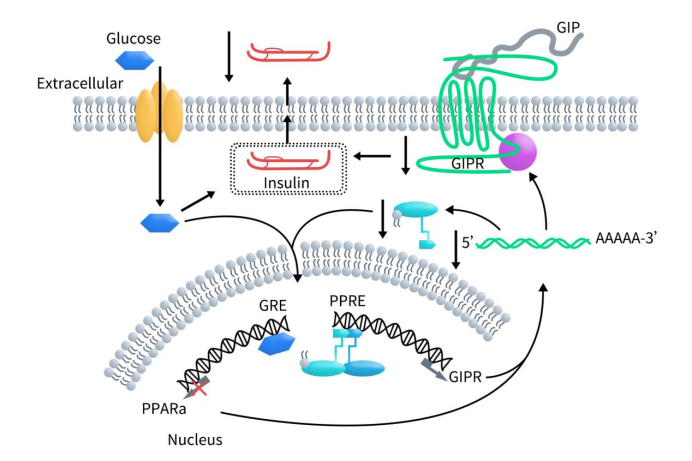
Figure 2. GIP binds to GIPR activating adenylyl cyclase, thereby potentiating glucose-induced insulin secretion [3]
Additionally, under normal blood glucose levels and hypoglycemic conditions, GIP binds to GIPR on pancreatic α-cells, promoting the secretion of glucagon. Besides its association with metabolic disorders, such as impaired postprandial insulin secretion in type 2 diabetes and the pathogenesis of obesity and related insulin resistance, several studies have also reported inappropriate activation of the GIP/GIPR axis in various endocrine tumors [4].
4. Progress in Clinical Research of GIPR-Targeted Drugs
Globally, there are currently 75 drugs targeting the GIPR, including 30 synthetic peptides, 10 small molecules, and nearly 44 dual-target drugs for both GIPR and GLP-1R. Of these, only 17 specifically target GIPR alone. Most of these drugs are in the preclinical stage, with only 28 in clinical development and just one approved for the market. The types of drugs include synthetic peptides, small molecules, chemical drugs, fusion proteins, bispecific antibodies, and recombinant peptides.
- Synthetic Peptides
Currently, there are 13 synthetic peptide drugs targeting GIPR in clinical use. Among these, the only FDA-approved GIPR-targeted drug is also a synthetic peptide: tirzepatide. There are two drugs in Phase III clinical trials: HRS-9531 from Shendi Pharmaceuticals, and retatrutide from Eli Lilly. HRS-9531 is a dual-target GIPR and GLP-1R agonist, while retatrutide is a triple-target agonist for GCGR, GIPR, and GLP-1R. In Phase II, there are five drugs: BGM-0504 from Borui Innovations, CT-388 and CT-868 from Roche, eHS20094 from Jiangsu Hosen, and VK-2735 from Viking. All five are dual-target GIPR and GLP-1R agonists. In Phase I, there are five drugs: DR10627 from Zhejiang Doral, HDM1005 from East China Pharmaceutical, LY-3493269 from Eli Lilly, THDBH120 from Dongbao Zixing, and UBT-251 from Federal Biologics. Except for UBT-251, which is a triple-target GCGR, GIPR, and GLP-1R agonist, the others are dual-target GIPR and GLP-1R agonists. These drugs are primarily aimed at treating obesity and diabetes.
| Drug | Targets | Indications | Company | Phase |
| Tirzepatide | GIPR x GLP-1R | Obesity, Type 2 Diabetes, Non-alcoholic Fatty Liver Disease | Lexaria Bioscience Corp., Eli Lilly & Co. | Approved |
| HRS-9531 | GIPR x GLP-1R | Obesity, Diabetes | Shendi Pharmaceuticals | Phase III |
| Retatrutide | GCGR x GIPR x GLP-1R | Atherosclerosis, Chronic Kidney Disease, Type 2 Diabetes | Eli Lilly & Co. | Phase III |
| BGM-0504 | GIPR x GLP-1R | Obesity, Diabetes | Borui Innovations | Phase II |
| CT-388 | GIPR x GLP-1R | Obesity, Type 2 Diabetes | Carmot Therapeutics, Inc., Roche Holding AG | Phase II |
| CT-868 | GIPR x GLP-1R | Type 1 Diabetes, Obesity | Roche Holding AG, Carmot Therapeutics, Inc. | Phase II |
| eHS20094 | GIPR x GLP-1R | Obesity, Type 2 Diabetes | Hansoh Pharmaceutical | Phase II |
| VK-2735 | GIPR x GLP-1R | Obesity, Metabolic Diseases, Non-alcoholic Fatty Liver Disease | Viking Therapeutics, Inc. | Phase II |
| DR10627 | GIPR x GLP-1R | Diabetes, Non-alcoholic Fatty Liver Disease | Doer Biotech | Phase I |
| HDM1005 | GIPR x GLP-1R | Obesity, Diabetes | Huadong Medicine Co.,Ltd | Phase I |
| LY-3493269 | GIPR x GLP-1R | – | Eli Lilly & Co. | Phase I |
| THDBH120 | GIPR x GLP-1R | Obesity, Type 2 Diabetes | Tonghua Dongbao Pharmaceutical | Phase I |
| UBT-251 | GCGR x GIPR x GLP-1R | Obesity, Type 2 Diabetes, Non-alcoholic Fatty Liver Disease | Federal Biologics | Phase I |
- Small Molecules
Currently, there are six small molecule drugs targeting GIPR in clinical development. Among them, Hanmi Pharmaceutical has two drugs: HM-15211 and HM-15275. Both are triple-target GCGR, GIPR, and GLP-1R agonists/modulators, with HM-15211 in Phase II and HM-15275 in Phase I. Eli Lilly has one drug, LY-3537021, which is a GIPR-specific agonist currently in Phase I. Additionally, HZ-012 from Zhejiang Heze, SCO-094 from Takeda, and KP-405 from Kariya are also in Phase I. All three are dual-target GIPR and GLP-1R agonists.
| Drug | Targets | Indications | Company | Phase |
| HM-15211 | GCGR x GIPR x GLP-1R | Non-alcoholic Fatty Liver Disease | Hanmi Pharmaceutical Co., Ltd. | Phase II |
| HM-15275 | GCGR x GIPR x GLP-1R | Obesity | Hanmi Pharmaceutical Co., Ltd. | Phase I |
| HZ-012 | GIPR x GLP-1R | Obesity | Heze Pharmaceutical | Phase I |
| LY-3537021 | GIPR | – | Eli Lilly & Co. | Phase I |
| SCO-094 | GIPR x GLP-1R | Diabetes, Non-alcoholic Fatty Liver Disease, Obesity | Takeda Pharmaceutical Co., Ltd. | Phase I |
| KP-405 | GIPR x GLP-1R | Parkinson’s Disease, Alzheimer’s Disease | Kariya Pharmaceuticals ApS | Early Phase I |
- Fusion Proteins & Bispecific Antibodies
Currently, there are two fusion proteins targeting GIPR in clinical development: MWN-101 from Shanghai Minwei, a GCGR x GIPR x GLP-1R agonist, and AMG-133 from Amgen, a GIPR x GLP-1R agonist. Both are in Phase II clinical trials. There is also one bispecific antibody in clinical development: GMA106, developed by Hongyun Huaning, which is a GIPR x GLP-1R agonist and is currently in Phase I.
| Drug Type | Drug | Targets | Indications | Company | Phase |
| Fusion Protein | MWN-101 | GCGR x GIPR x GLP-1R | Type 2 Diabetes, Obesity | Shanghai Minwei Biotechnology | Phase II |
| Fusion Protein | AMG-133 | GIPR x GLP-1R | Type 2 Diabetes, Obesity | Amgen, Inc. | Phase II |
| Bispecific Antibody | GMA106 | GIPR x GLP-1R | Diabetes, Pulmonary Sarcoidosis, Non-alcoholic Fatty Liver Disease, Obesity | Hongyun Huaning Biopharma, China National Biotec Group, Gmax Biopharm Australia Pty Ltd. | Phase I |
- Recombinant Peptides
Currently, there are three recombinant peptide drugs targeting GIPR in clinical development: NNC0519-0130 from Novartis, LY-3532226 from Eli Lilly, and GIP[3-30]NH2 from Gentofte. The most advanced is Novartis’s NNC0519-0130, which is in Phase II. The other two drugs are in Phase I and Early Phase I, respectively. Notably, GIP[3-30]NH2 from Gentofte is currently the only clinical candidate that acts exclusively as a GIPR antagonist.
| Drug | Targets | Indications | Company | Phase |
| NNC0519-0130 | GIPR x GLP-1R | Type 2 Diabetes, Obesity | Novo Nordisk A/S | Phase II |
| LY-3532226 | GIPR | Diabetes, Hypoglycemia | Eli Lilly & Co. | Phase I |
| GIP[3-30]NH2 | GIPR | Obesity | Gentofte Hospital | Early Phase I |
- Other Drug Types
In addition, there are four other drugs targeting GIPR currently in clinical development: LBT-6030 from Longevity, 3BP-3775 from 3B Pharmaceuticals, GLP-1/GIP/Glucagon Tri-agonist from Eli Lilly, and NN-9541 from Novartis, all of which are in Phase I. Notably, LBT-6030 from Longevity is a dual-target GIPR x GLP-1R agonist, modified using Longevity’s Hybridtide® technology. This technology significantly enhances the durability and stability of the molecules compared to traditional peptides. Hybridtides® can effectively mimic the structure of natural peptides and small proteins, improving overall product characteristics while retaining potency and specificity. Additionally, LBT-6030 has been optimized using Longevity’s oral peptide platform.
| Drug Type | Drug | Targets | Indications | Company | Phase |
| Biologic | LBT-6030 | GIPR x GLP-1R | Parkinson’s Disease | Longevity Biotech, Inc. | Phase I |
| Diagnostic Radiopharmaceutical | 3BP-3775 | GIPR | Neuroendocrine Tumors | 3B Pharmaceuticals GmbH | Early Phase I |
| Chemical | GLP-1/GIP/Glucagon Tri-agonist | GCGR x GIPR x GLP-1R | Diabetes | Eli Lilly & Co. | Phase I |
| Chemical | NN-9541 | GIPR x GLP-1R | Type 2 Diabetes | Novo Nordisk Pharmaceuticals Pty Ltd. | Phase I |
5. DIMA’s GIPR-Targeted Products Enhance Drug Development
DIMA Biotech is a biotechnology company specializing in preclinical development products and services for druggable targets. We now offer a comprehensive range of products and services related to the GIPR target. Our offerings include active proteins, reference antibodies, and flow cytometry validation monoclonal antibodies. Additionally, we provide various services such as custom antibody production for different species, antibody humanization, and affinity maturation. To accelerate the development of GIPR-based biologics, DIMA has established a single B-cell library for the GIPR target, enabling the generation of lead antibody candidates in as little as 28 days. We have identified 15 promising GIPR lead molecules, which are available for immediate functional assessment. For more information, please feel free to inquire.
- GIPR Protein&Antibody
| Product Type | Catalog Number | Product Name |
| Full-Length Active Protein | FLP100130 | Human GIPR Full-Length Protein – Synthetic Nanodisc |
| ECD Recombinant Protein | PME101267 | Human GIPR Protein, hFc Tag |
| PME-M100109 | Mouse GIPR Protein, hFc Tag | |
| Flow Cytometry Validation Antibody | DMC101001 | Anti-GIPR Antibody (3G5); IgG1 Chimeric mAb |
| Reference Antibody | BME100209 | Anti-GIPR (Maridebart Biosimilar) mAb |
| Biotinylated Antibody | DMC101001B | Biotinylated Anti-GIPR Antibody (3G5); IgG1 Chimeric mAb |
| BME100209B | Biotinylated Anti-GIPR (Maridebart Biosimilar) mAb |
- GIPR Lead mAb Molecule Research Progress

Reference:
[1]Hauser, A., Attwood, M., Rask-Andersen, M. et al. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov 16, 829–842 (2017).
[2]Yang, D., Zhou, Q., Labroska, V. et al. G protein-coupled receptors: structure- and function-based drug discovery. Sig Transduct Target Ther 6, 7 (2021).
[3]Lynn FC, Thompson SA, Pospisilik JA, Ehses JA, Hinke SA, Pamir N, McIntosh CH, Pederson RA. A novel pathway for regulation of glucose-dependent insulinotropic polypeptide (GIP) receptor expression in beta cells. FASEB J. 2003 Jan;17(1):91-3.
[4]Regazzo D, Barbot M, Scaroni C, Albiger N, Occhi G. The pathogenic role of the GIP/GIPR axis in human endocrine tumors: emerging clinical mechanisms beyond diabetes. Rev Endocr Metab Disord. 2020 Mar;21(1):165-183.
