In 2024, the radiopharmaceutical sector has seen a flurry of activity in mergers and acquisitions. In March, AstraZeneca announced a $2.4 billion acquisition of Fusion Pharmaceuticals, a biopharmaceutical company focused on developing next-generation radiolabeled conjugates (RDCs). In April, Novartis invested nearly $2.71 billion to partner with PeptiDream to co-develop several macrocyclic-targeted conjugate radiopharmaceuticals. In May, Novartis made headlines again by acquiring Mariana Oncology for a $1 billion upfront payment, gaining access to its innovative pipeline of radiopharmaceuticals. That same month, Eli Lilly entered a multi-target research collaboration with Aktis Oncology for $1.1 billion, leveraging Aktis’s novel mini-protein technology platform to develop cancer-targeting radiopharmaceuticals. In June, Eli Lilly partnered with Radionetics to adopt its small molecule GPCR-targeting radiopharmaceutical technology, securing exclusive rights to acquire Radionetics for $1 billion. In September, Sanofi invested $356 million in a licensing agreement with radiopharmaceutical companies RadioMedix and OranoMed for AlphaMedix…
News continues to emerge about the major pharmaceutical companies’ investments in radiopharmaceuticals, making this sector a competitive battleground for industry giants. But what exactly are radiopharmaceuticals? What are the current popular targets and the indications they address?
1. Introduction to Radiopharmaceuticals
Radiopharmaceuticals, also known as radioactive drugs or radioisotope-conjugated drugs, are a special class of medications that contain radioactive isotopes for medical diagnosis and treatment. Based on their applications, radiopharmaceuticals can be divided into diagnostic and therapeutic categories.
Diagnostic radiopharmaceuticals utilize radioactive isotopes for medical imaging, primarily including two types: those used for organ imaging and those for functional assessment. These agents can be combined with gamma cameras (such as PET or SPECT) to study the function, biochemical metabolism, and abnormal gene expression of affected tissues at the molecular level, enabling rapid, non-invasive, and real-time imaging of physiological and pathological processes.
Therapeutic radiopharmaceuticals are administered to patients either orally or via injection. These drugs are designed to selectively concentrate in diseased tissues, where the emitted radiation from the decaying isotopes can damage and destroy tumor cells.
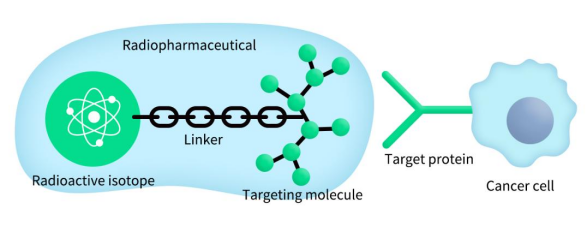
Figure 1. The general structure of radiopharmaceuticals
In recent years, new targets such as Prostate-Specific Membrane Antigen (PSMA), Fibroblast Activation Protein (FAP), and Human Epidermal Growth Factor Receptor 2 (HER2) have gained prominence in the field of Radioligand Therapy (RLT), accelerating the development of therapeutic radiopharmaceuticals. So, which targets are currently in high demand within the radiopharmaceutical sector? What is the latest research progress on the related drugs?
2. “Hot” Targets in Radiopharmaceuticals
According to incomplete statistics, there are a total of 801 radiopharmaceuticals globally, with 20 approved for market use. The combined number of drugs in Phase 1 and Phase 2 clinical trials reaches 149, indicating a high feasibility in this sector. However, there are also 204 drugs that have been stalled, paused, or terminated. Additionally, 289 drugs are in the preclinical stage, showing that pharmaceutical companies are actively pursuing new drug development.
In terms of targets, current global drug development is primarily focused on PSMA, FAP, and HER2, with 26 drugs targeting PSMA in various clinical stages. The development of PSMA and FAP-targeting drugs is relatively advanced, and some have already received approval. In contrast, there are fewer drugs in development for targets like SSTR, SSTR2, and PDL1, with most still in preclinical or early-stage trials. Below, we outline the research progress of drugs targeting these popular pathways in clinical phases.
2.1 PSMA
Prostate-Specific Membrane Antigen (PSMA), also known as Glutamate Carboxypeptidase II (GCPII), is a transmembrane glycoprotein found on the surface of cells, exhibiting both carboxypeptidase and folate hydrolase activity. PSMA is expressed at very low levels in normal prostate and non-prostate tissues but is found at levels 100 to 1,000 times higher in prostate cancer tissues. This makes PSMA a key target for imaging diagnosis and targeted radionuclide therapy, as well as immunotherapy for prostate cancer and its metastases.
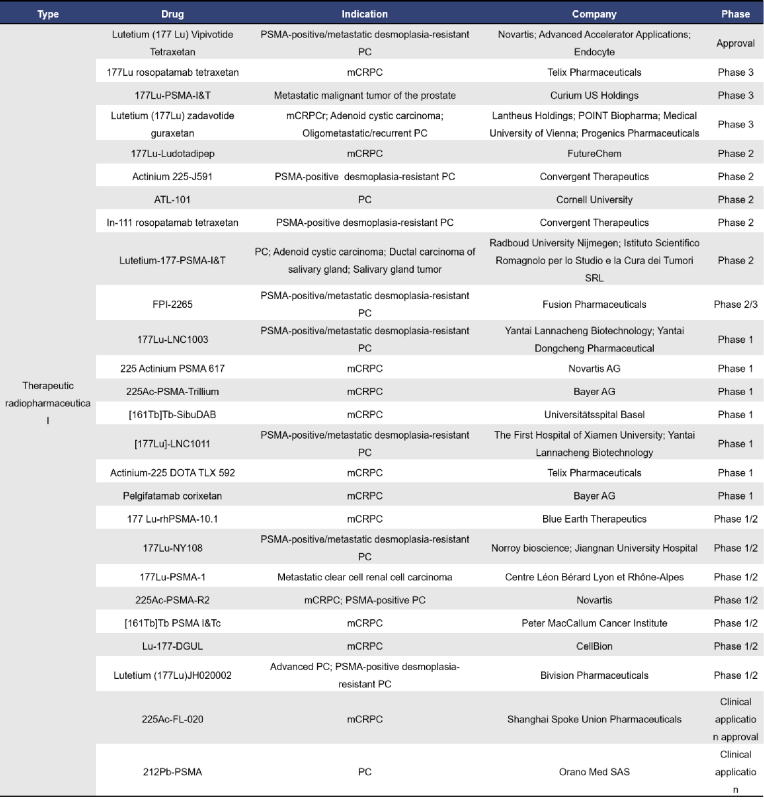
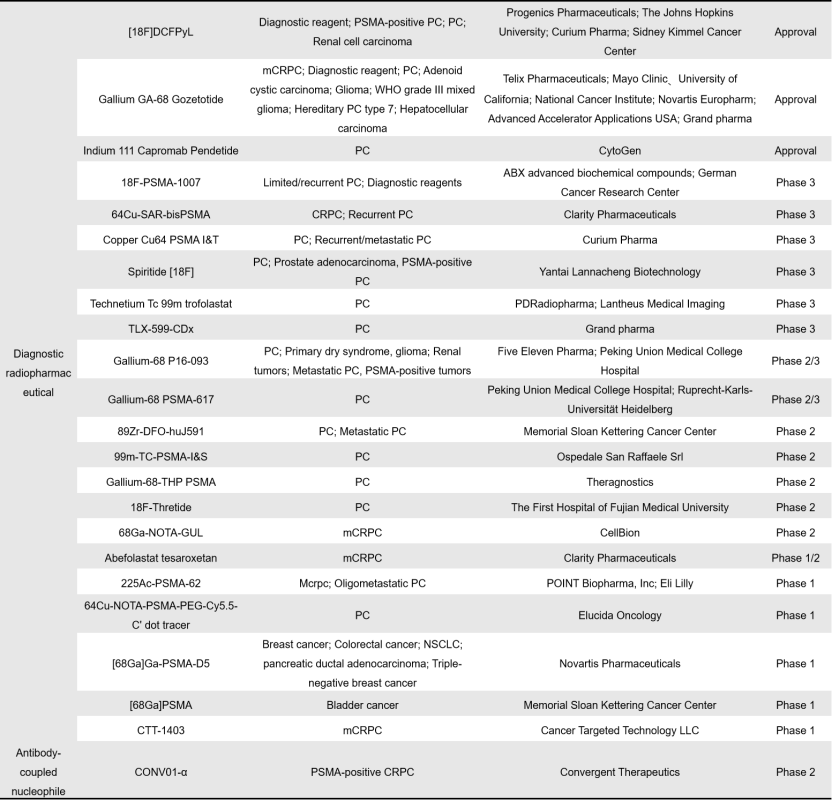
Currently, there are a total of 90 radiopharmaceuticals targeting PSMA globally, with 43 in clinical stages and 4 already approved for market use. Among the clinical and approved drugs, 26 are therapeutic radiopharmaceuticals, while 22 are diagnostic radiopharmaceuticals.
Therapeutic radiopharmaceuticals showcase their power through Novartis’s Lutetium (177Lu) Vipivotide Tetraxetan, approved in March 2022 in the United States for treating PSMA-positive metastatic castration-resistant prostate cancer (mCRPC), and later in December in the European Union. This drug’s active component, Lutetium-177, binds to PSMA-expressing cells. The β-negative radiation from Lutetium-177 then targets these cells and nearby tissues, causing DNA damage and cell death.
On the diagnostic front, CytoGen’s Indium-111 Capromab Pendetide stands out as a pioneer. Approved in October 1996 in the United States, it was the first commercialized anti-PSMA antibody. Unlike therapeutic counterparts, this antibody can only bind to the intracellular epitope of PSMA.
2.2 FAP
Fibroblast Activation Protein (FAP) is a serine oligopeptidase that is primarily expressed during developmental stages and is rarely found in healthy adult tissues. However, FAP is highly upregulated in pathological tissues, particularly at sites of remodeling in conditions such as cancer, fibrosis, arthritis, wounds, or inflammation, especially on fibroblasts.
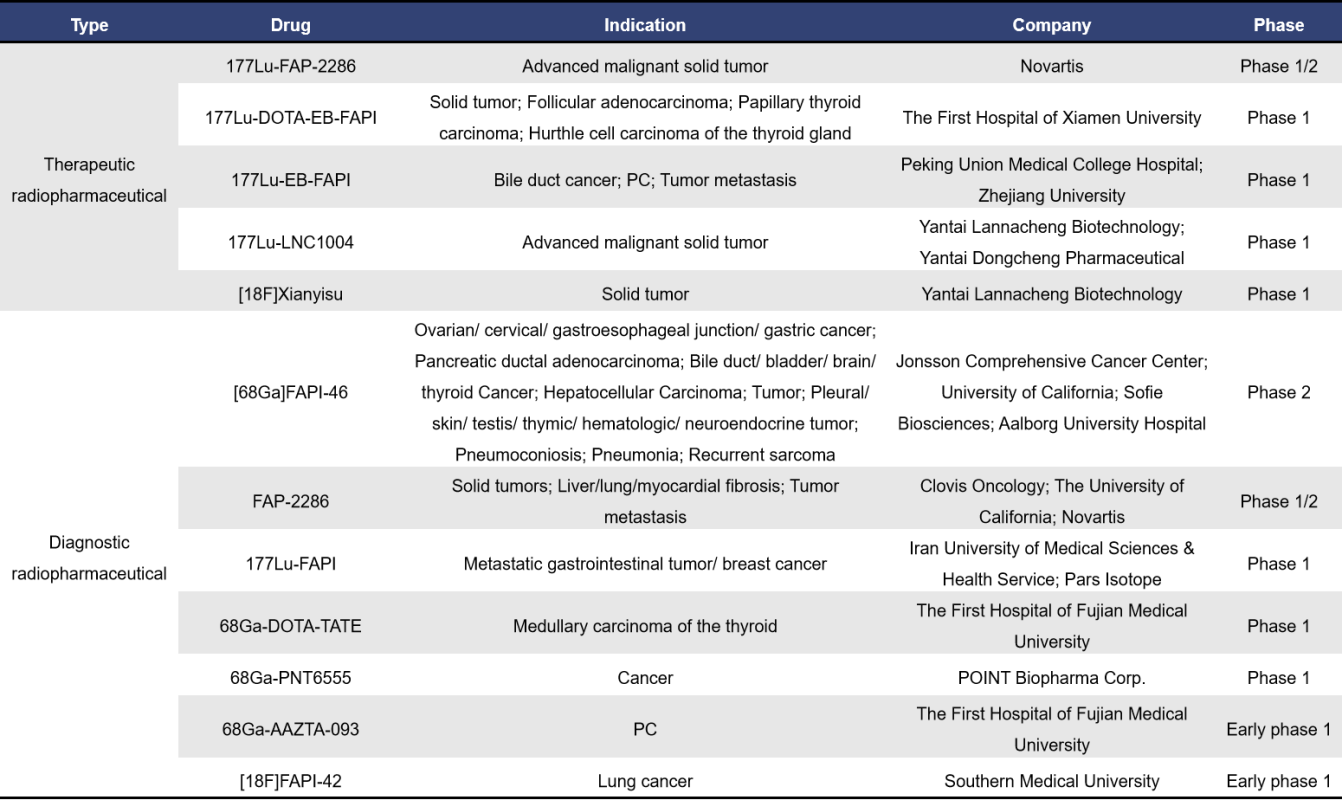
Currently, there are a total of 44 radiopharmaceuticals targeting FAP globally, with 12 in clinical stages and nearly 28 in preclinical development. Among the clinical stage drugs, 5 are therapeutic radiopharmaceuticals and 7 are diagnostic radiopharmaceuticals.
A representative therapeutic radiopharmaceutical is Novartis’s 177Lu-FAP-2286, originally developed by Clovis Oncology, which was acquired by Novartis at the end of 2022. 177Lu-FAP-2286 is the first peptide-targeted radioligand therapy, composed of a targeting peptide that binds to FAP and a site for attaching a radioactive isotope for imaging and therapeutic purposes. It is currently in the advanced stages of clinical development (Phase 1/2, NCT04939610). A key diagnostic radiopharmaceutical example is [68Ga]FAPI-46.
2.3 HER2
HER2 is a tyrosine kinase receptor membrane glycoprotein encoded by the ERBB2 gene. It is a member of the epidermal growth factor receptor (EGFR) family, alongside EGFR, HER3, and HER4. Abnormal HER2 expression is one of the most common phenomena in malignant tumors, particularly prevalent in breast cancer.
Currently, there are a total of 40 radiopharmaceuticals targeting HER2 globally, with 11 in clinical stages and nearly 15 in preclinical development. Among the clinical stage drugs, 3 are therapeutic radiopharmaceuticals, while 8 are diagnostic radiopharmaceuticals.
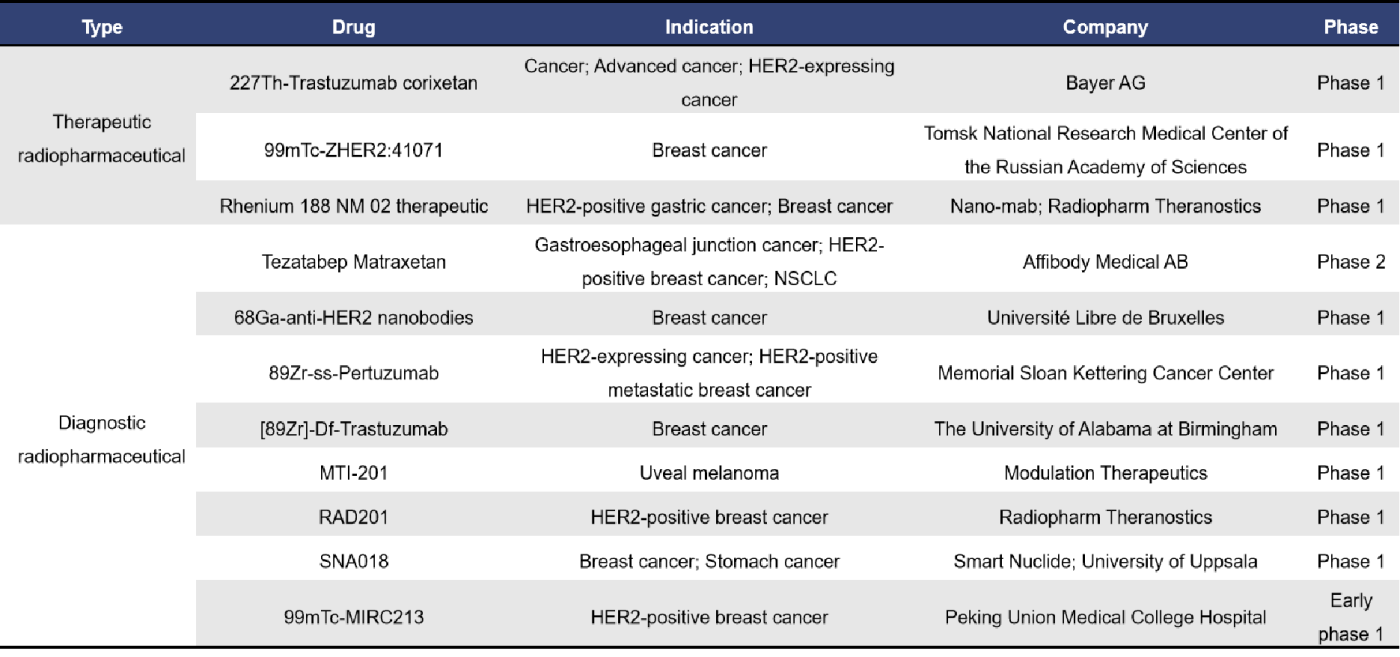
2.4 SSTR and SSTR2
Somatostatin receptors (SSTR) belong to the G protein-coupled receptor (GPCR) family. To date, researchers have cloned and identified five SSTR subtypes: SSTR1, SSTR2, SSTR3, SSTR4, and SSTR5. SSTRs are expressed in various normal tissues and are also highly expressed in multiple tumor types. Studies have shown that tumor cells from different tissues can express a variety of SSTRs, with SSTR2 being the most abundantly expressed.
Currently, there are a total of 23 radiopharmaceuticals targeting SSTR and SSTR2, amounting to 46 drugs overall. Among these, 5 have received market approval, 1 is in the application stage for approval, 14 are in clinical stages, and 8 are in preclinical development.
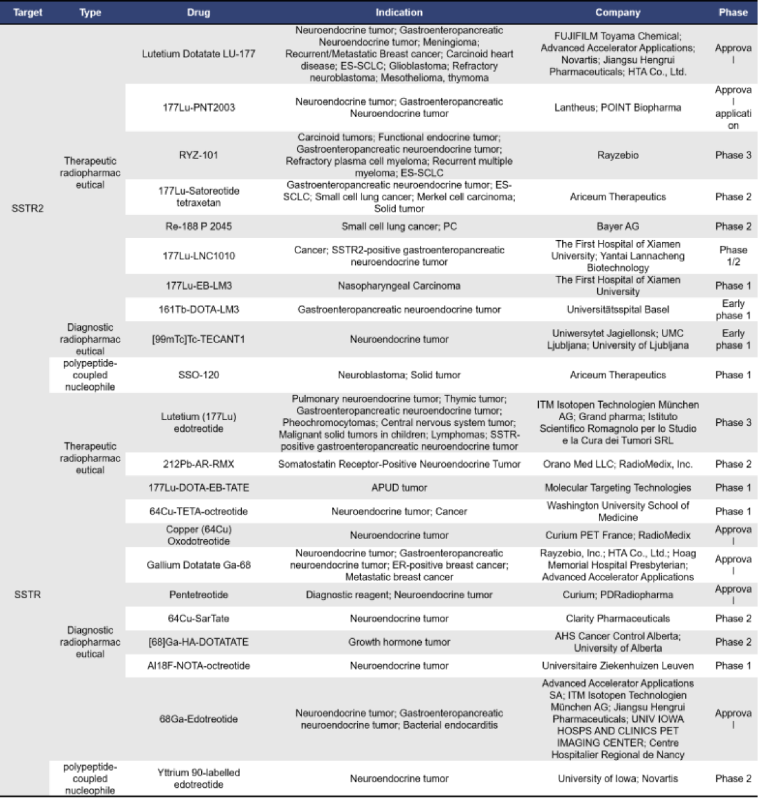
2.5 PDL1
Programmed Death Ligand 1 (PDL1), also known as B7H1 or CD274, is highly expressed on the surface of cancer cells. It binds to T cells, creating the illusion that cancer cells are “harmless,” which in turn reduces T cell activity and allows cancer cells to evade immune detection.
Currently, there are 22 radiopharmaceuticals targeting PDL1 globally, with 7 in clinical stages and 12 in preclinical development.
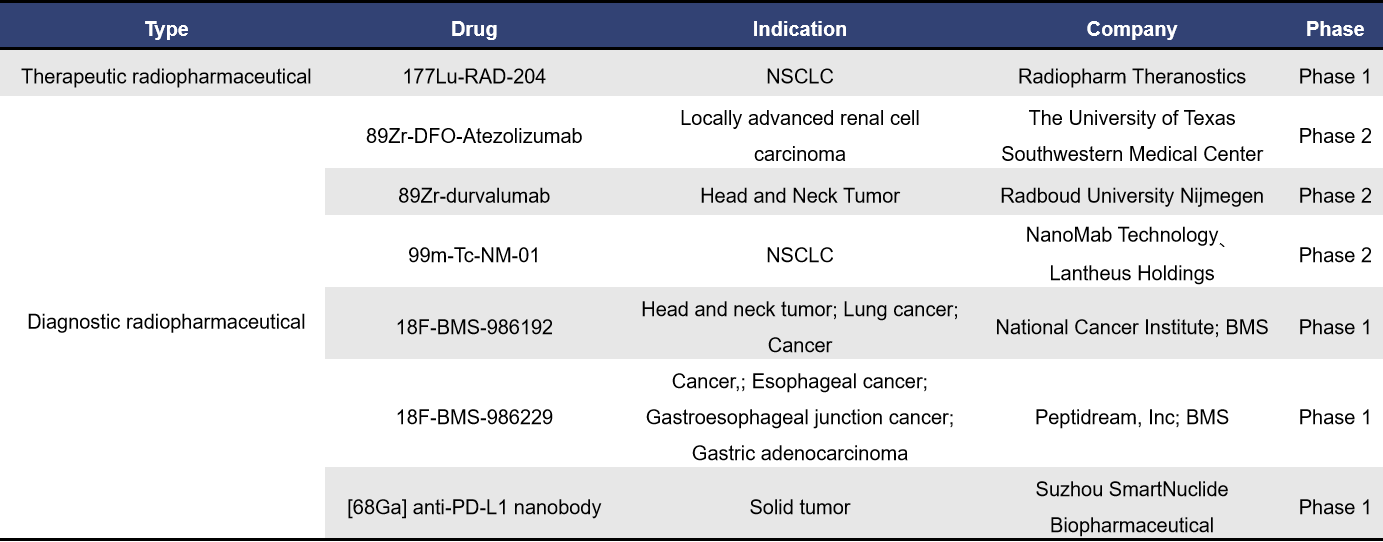
2.6 CAIX
Carbonic Anhydrase IX (CAIX), also known as CA9, is a transmembrane protein that is specifically overexpressed on the surface of hypoxic tumor cells. It plays a crucial role in regulating the acid-base balance within and outside tumor cells, closely associated with tumor proliferation, invasion, and metastasis. Therefore, CAIX is considered a promising target for tumor imaging and therapy.
Currently, there are 16 radiopharmaceuticals targeting CAIX globally, with 1 in the application stage for market approval, 5 in clinical stages, and 2 in preclinical development.
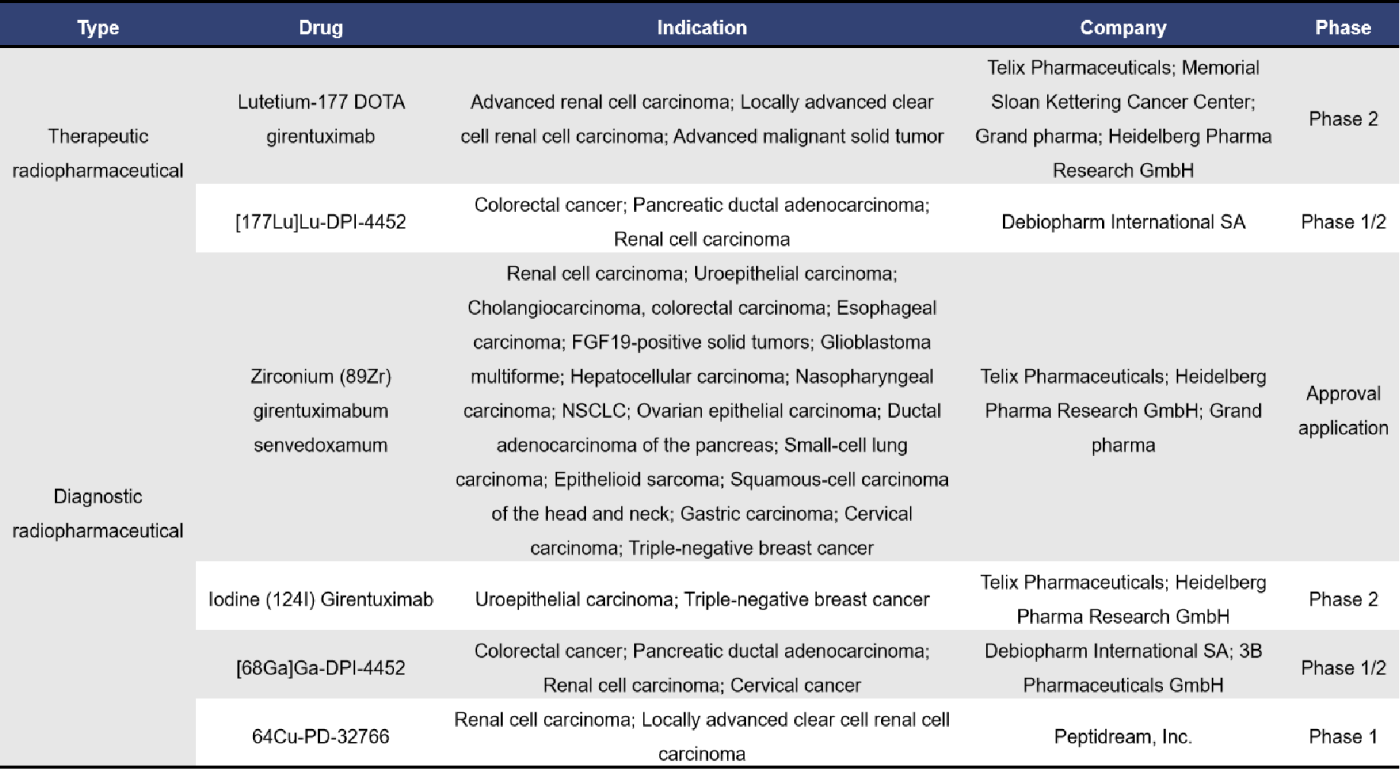
In addition to the above targets, several established tumor targets have also been integrated into radiopharmaceutical development, including EGFR, GRPR, MUC1, CD38, CD20, GLP1R, HER3, and TROP2. Among these, the target with the highest number of drugs entering clinical stages is GRPR, while most of the other targets have radiopharmaceuticals primarily in the preclinical phase.
3. Lead Molecules from DIMA Bio Support Radiopharmaceutical Development
DIMA Biotechnology is a biotechnology company focused on providing preclinical research products and services for druggable targets. In addition to offering recombinant proteins and antibody products related to specific targets, DIMA Bio provides tailored services for various species-specific antibodies, antibody humanization, and affinity maturation.
To accelerate the development of radiopharmaceuticals, DIMA Bio has established a single B-cell seed bank for targeted molecules, enabling the rapid generation of lead antibody candidates in as little as 28 days. Additionally, we have identified several lead molecules for key targets and have verified their cross-reactivity with human and monkey proteins. Clients can receive these molecules the next day for functional evaluation. For more information on additional lead molecule targets, please refer to our lead molecule catalog.
