On September 17, a collaboration between Daiichi Sankyo and Merck announced that patritumab deruxtecan, a potentially first-in-class antibody-drug conjugate (ADC) targeting HER3, has met its primary endpoint for progression-free survival (PFS) in the Phase 3 lung cancer trial HERTHENA-Lung02. This treatment demonstrated statistically significant improvement compared to platinum-based chemotherapy combined with pemetrexed induction and pemetrexed maintenance therapy. This finding further validates the druggability of the HER3 target in lung cancer treatment. So, what is the current clinical progress of HER3-targeting drugs, and which pharmaceutical companies are positioning themselves in this space?
1. Structure of HER3/ERBB3
Human epidermal growth factor receptor 3 (HER3), also known as receptor tyrosine-protein kinase ERBB3, is a membrane-bound protein that belongs to the ERBB/HER family of receptor tyrosine kinases (RTKs). In mammals, there are four ERBB/HER receptors: EGFR (ERBB1/HER1), HER2 (ERBB2), HER3 (ERBB3), and HER4 (ERBB4). Numerous studies have shown that ERBB/HER receptors play a crucial role in animal development, and alterations in their function can lead to the pathophysiological development of certain types of tumors [1][2]. HER3 has garnered increasing attention due to its unique role in the pathogenesis of non-small cell lung cancer (NSCLC) and its impact on patient prognosis.
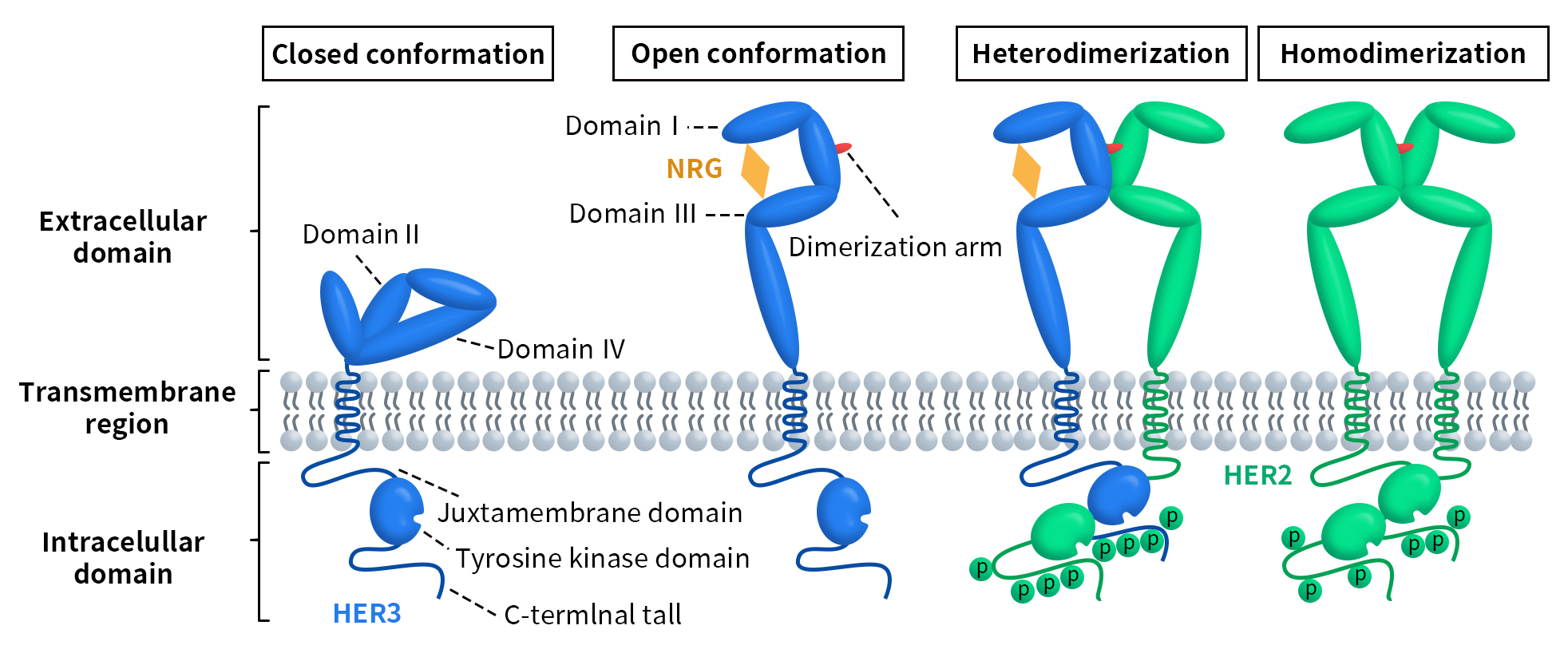
HER3 was discovered by Kraus et al. in 1989 and is encoded by the ERBB3 gene located on chromosome 12q13 in humans [3]. This gene consists of 23,651 base pairs and encodes the HER3 protein, which contains 1,342 amino acids. Like other members of the ERBB receptor tyrosine kinase family, HER3 has an extracellular domain, a transmembrane domain, and an intracellular domain. The extracellular domain is further divided into four subdomains (I-IV). Subdomains I and III are rich in leucine and primarily participate in ligand binding. Subdomains II and IV are rich in cysteine, likely facilitating protein conformation and stability through the formation of disulfide bonds. Subdomain II also contains the dimerization loop necessary for dimer formation. The intracellular domain consists of a juxtamembrane region, a tyrosine kinase domain, and a C-terminal tail with phosphorylatable residues [4] [5].
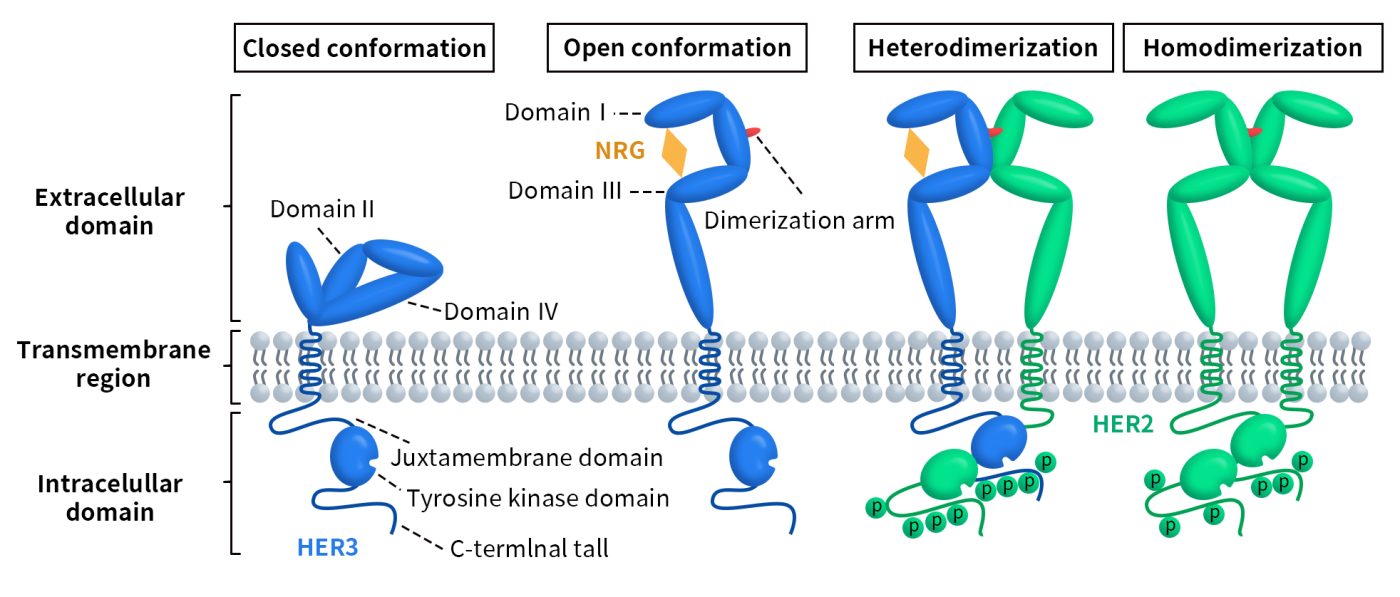
Figure 1. Schematic representation of the structural changes and activation of ErbB/HER receptors [6]
HER3 can bind to the ligands heregulin and neuregulin 2 (NRG2). As shown in Figure 1, in the absence of a ligand, direct intramolecular interactions between subdomains II and IV keep HER3 in an inactive conformation. When a ligand binds to subdomains I and III, it triggers a conformational change in the extracellular domain of HER3, exposing the dimerization arm located in subdomain II. This dimerization arm allows HER3 to interact with another member of the ERBB receptor tyrosine kinase family or with another HER3 monomer, forming either heterodimers or homodimers [7][8].
2. HER3/ERBB3 Signaling Pathway
HER3 is widely expressed in various human adult tissues, including the gastrointestinal tract, urinary system, respiratory system, reproductive system, skin, endocrine system, and nervous system. Unlike other members of the ERBB receptor tyrosine kinase family, which can be activated through autophosphorylation upon ligand binding, HER3 has a kinase deficiency. Its autophosphorylation activity is only 1/1000th that of EGFR, and it lacks the ability to phosphorylate other proteins [9]. As mentioned earlier, the extracellular domain of HER3 adopts an active conformation upon ligand binding. Due to its kinase deficiency, HER3 can only undergo weak autophosphorylation. However, HER3 can form heterodimers with other receptor tyrosine kinases (RTKs), effectively facilitating the phosphorylation of its cytoplasmic domain. The primary dimerization partner of HER3 is HER2. When heterodimers are formed, both the MAPK and AKT signaling pathways are activated. This activation regulates genes involved in various cellular responses, such as cell cycle control, proliferation, survival, metabolism, apoptosis, and angiogenesis. Additionally, HER3 itself is subject to regulation at different levels; for example, two ubiquitin ligases, NEDD4 and NRDP1, can mediate its ubiquitination and proteasomal degradation. The AKT-regulated deubiquitinating enzyme USP8 can negatively regulate NRDP1. Furthermore, activated androgen receptors (AR) control HER3 levels by binding to the promoter region of NRDP1 and activating its transcription.
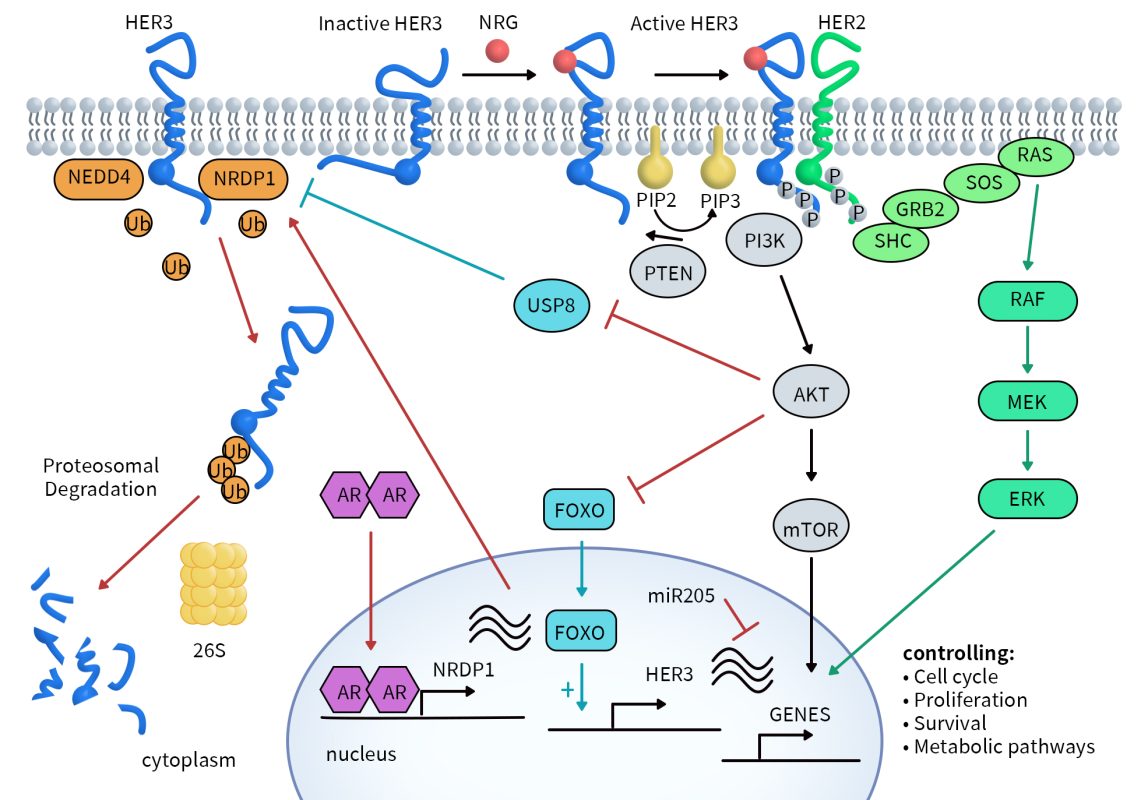
Figure 2. Regulation of HER3 and downstream signaling [10]
3. Clinical Research Progress on HER3-Targeted Drugs
While there is no evidence that HER3 overexpression, constitutive activation, or mutations directly cause cancer, its role as a dimerization partner of HER2 is linked to growth, proliferation, chemotherapy resistance, and the promotion of invasion and metastasis. Currently, HER3 expression or overexpression has been identified in various tumor types, including breast cancer, ovarian cancer, lung cancer, colorectal cancer, pancreatic cancer, melanoma, gastric cancer, and head and neck cancer. There are a total of 113 HER3-targeted drugs, with the highest number (56) in the preclinical stage. The numbers for clinical phase 2 and phase 1 drugs are smaller, at 8 and 7, respectively. However, there is one drug that has received marketing approval and one that has been granted clinical trial authorization, indicating the potential viability of this therapeutic pathway. The types of HER3-targeted drugs in clinical development are also diverse, including ADCs, monoclonal antibodies, bispecific antibodies, and vaccines, with ADCs holding a dominant position.
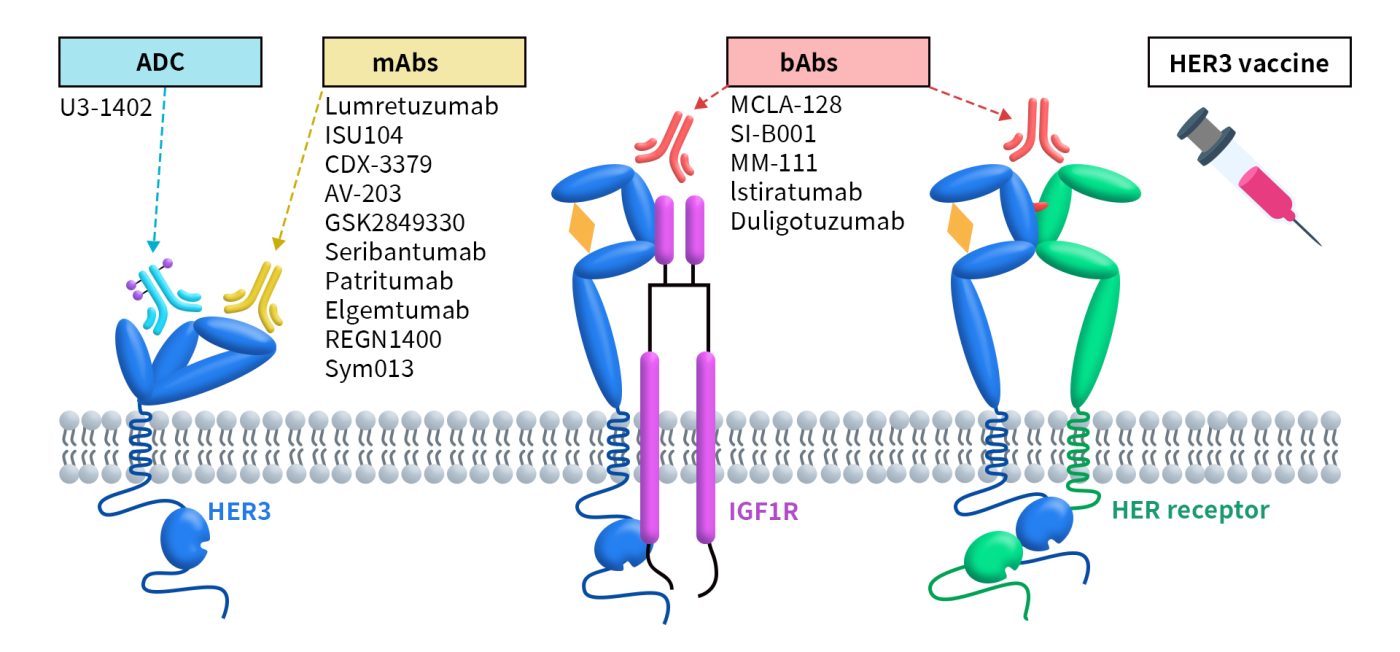
Figure 3. Therapies against HER3 under clinical development [6]
3.1 HER3 ADC Drugs
Currently, there are approximately 43 ADCs targeting HER3 globally. Among these, 62.7% (27 drugs) are in the preclinical stage, while 23% (10 drugs) are in clinical stages. As of now, there are no approved drugs on the market, with the highest development stage being phase 3 clinical trials. The details of these drugs and the companies involved are shown in the table below:
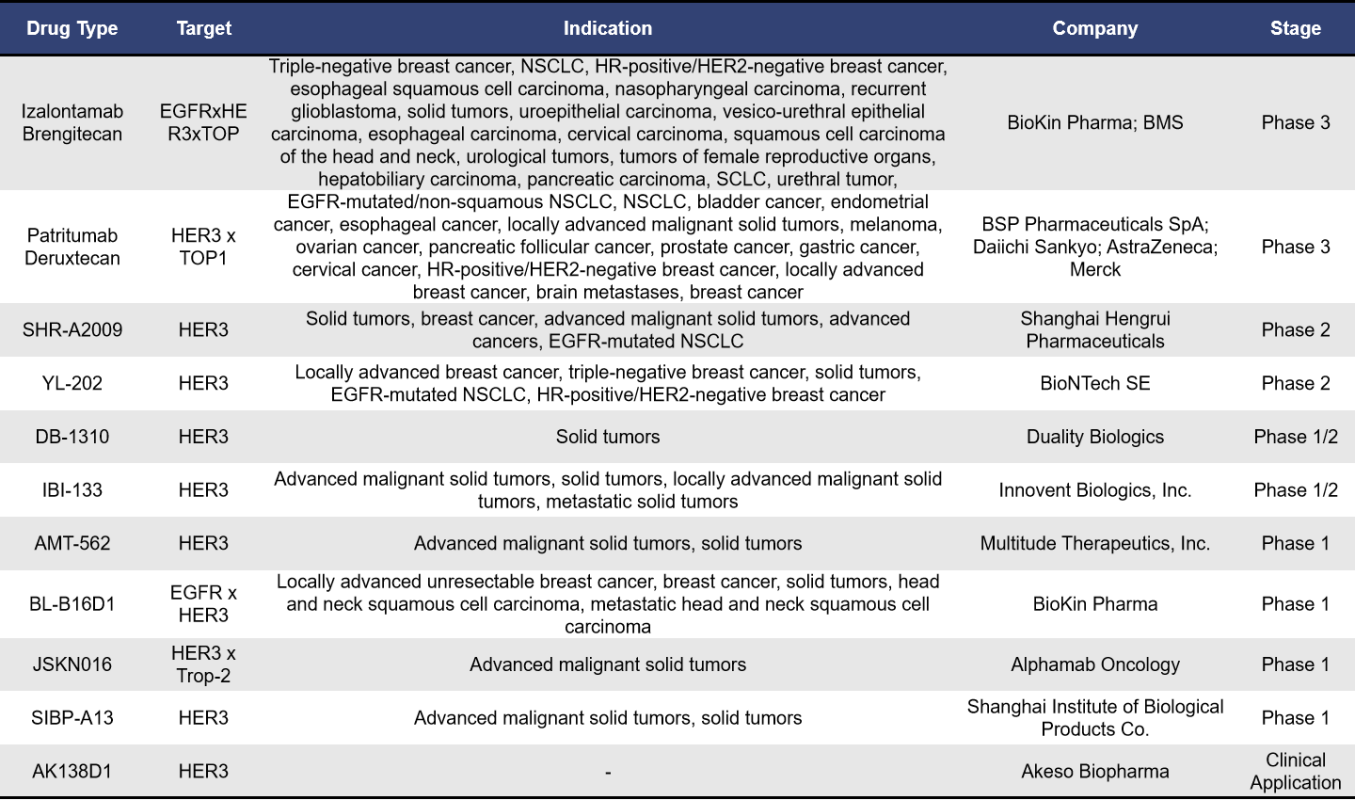
- Izalontamab Brengitecan
Izalontamab Brengitecan, also known as BL-B01D1, is an ADC developed by SystImmune, a subsidiary of BioKin Pharma. This drug is the world’s first dual-target EGFR and HER3 ADC to enter clinical trials, featuring a drug-to-antibody ratio (DAR) between 7.5 and 8. The tetravalent BL-B01D1 contains two binding domains for different growth factor receptors, enabling it to drive cancer cell proliferation and survival. BL-B01D1 effectively blocks the signals from EGFR and HER3 to cancer cells, thereby reducing proliferation and survival signals. After internalization mediated by the antibody, BL-B01D1 is transported to the cancer cell lysosome, where it releases its therapeutic payload, inducing genotoxic stress pathways that lead to cancer cell death. In December 2023, BioKin Pharma licensed BL-B01D1 to BMS for an upfront payment of $800 million, with a potential total value of up to $8.4 billion. Under the agreement, BMS is exclusively responsible for developing and commercializing of BL-B01D1 in all regions outside mainland China.
Currently, BL-B01D1 has initiated seven phase 3 clinical trials, including:
BL-B01D1-301 (NCT06382116): Targeting locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC) with EGFR-sensitive mutations, comparing against platinum-based chemotherapy after EGFR-TKI treatment. BL-B01D1-302 (NCT06382129): For locally advanced or metastatic EGFR wild-type NSCLC, comparing against docetaxel after anti-PD-1/PD-L1 monoclonal antibody and platinum chemotherapy. BL-B01D1-303 (NCT06118333): Targeting recurrent or metastatic nasopharyngeal carcinoma, comparing against chemotherapy (docetaxel, capecitabine, gemcitabine) after PD-1/PD-L1 monoclonal antibody as last-line treatment plus at least two lines of platinum chemotherapy. BL-B01D1-304 (NCT06500026): For recurrent small cell lung cancer, comparing against topotecan after anti-PD-1/PD-L1 monoclonal antibody and platinum chemotherapy. BL-B01D1-305 (NCT06304974): Targeting recurrent or metastatic esophageal cancer, comparing against chemotherapy (irinotecan, paclitaxel, docetaxel) after failure of PD-1/PD-L1 plus platinum chemotherapy. BL-B01D1-306 (NCT06343948): For locally advanced recurrent or metastatic HR+/HER2- breast cancer, comparing against chemotherapy (eribulin, vinorelbine, capecitabine, gemcitabine) after at least one line of chemotherapy. BL-B01D1-307 (NCT06382142): Targeting unresectable locally advanced or metastatic triple-negative breast cancer, comparing against chemotherapy (eribulin, vinorelbine, capecitabine, gemcitabine) after treatment with taxane-based agents.
- Patritumab Deruxtecan (U3-1402)
Patritumab Deruxtecan, also known as U3-1402, is an antibody-drug conjugate (ADC) targeting HER3, developed by Daiichi Sankyo. U3-1402 consists of a fully humanized anti-HER3 IgG1 monoclonal antibody covalently linked to a topoisomerase I inhibitor via a cleavable peptide linker, with a drug-to-antibody ratio (DAR) of 7.8. This ADC is currently being co-developed and commercialized by Daiichi Sankyo and Merck.
On September 17, 2024, Merck and Daiichi Sankyo announced that the phase III HERTHENA-Lung02 study (NCT05338970) of patritumab deruxtecan in patients with locally advanced or metastatic EGFR mutation-positive non-small cell lung cancer (NSCLC) who had previously received EGFR-TKI treatment met its primary endpoint for progression-free survival (PFS). Compared to patients receiving platinum-based chemotherapy followed by pemetrexed maintenance, those treated with patritumab deruxtecan exhibited statistically significant improvements in PFS.
Earlier, in December 2023, the FDA accepted the biologics license application (BLA) for patritumab deruxtecan based on the phase II HERTHENA-Lung01 study and granted it priority review designation for the treatment of adults with locally advanced or metastatic EGFR mutation-positive NSCLC who had received two or more prior systemic therapies. However, in June 2024, Daiichi Sankyo and Merck received a complete response letter (CRL) from the FDA regarding the BLA, indicating that the approval was delayed due to issues related to third-party manufacturing facilities.
In addition to the previously mentioned ADCs, there are several other HER3-targeting antibody-drug conjugates in development. These include SHR-A2009 from Hengrui Pharmaceutical, YL202 from Yilian Biotech, DB-1310 from Duality Biologics, and IBI133 from Innovent Biologics. Notably, SHR-A2009 received FDA Fast Track designation in January 2024 for the treatment of metastatic NSCLC with EGFR mutations that progressed after treatment with third-generation EGFR tyrosine kinase inhibitors and platinum-based chemotherapy.
3.2 HER3 Bispecific Antibodies
As of now, there are approximately 12 bispecific antibodies targeting HER3 globally. Among of them, there are 5 antibodies in the preclinical phase, 1 antibody in the application stage for market approval, and 1 antibody in Phase 3.
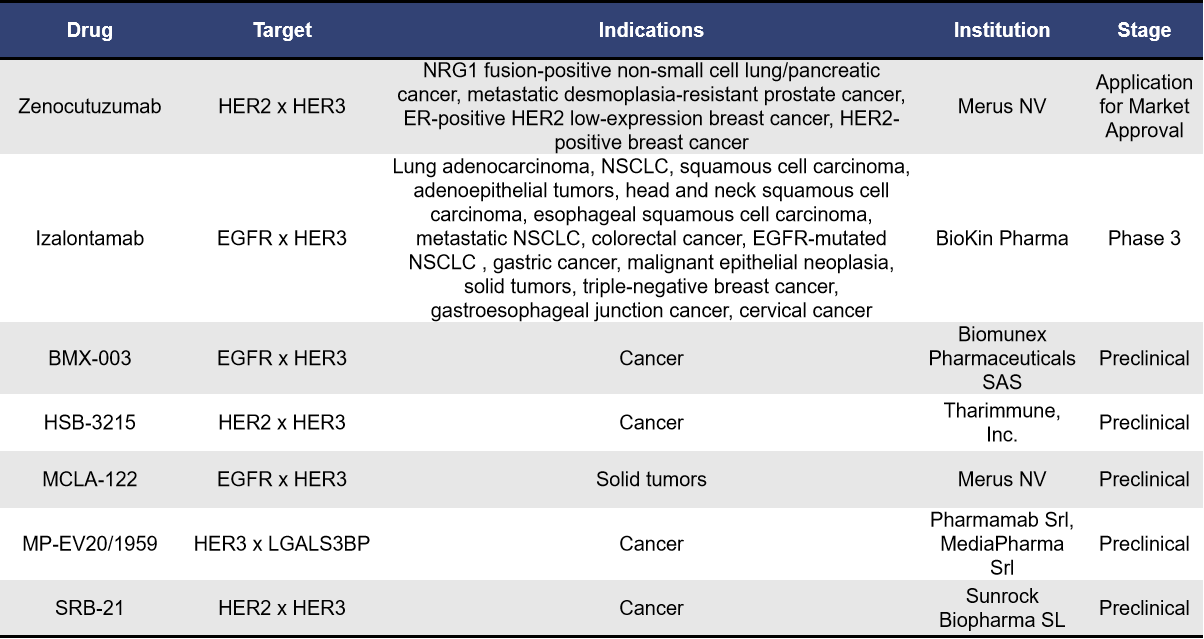
- Zenocutuzumab
Zenocutuzumab, also known as Zeno or MCLA-128, is a bispecific antibody developed by Merus NV that targets both HER2 and HER3. This dual-action antibody primarily works by specifically binding to HER2, effectively blocking the interaction between HER3 and NRG1 or NRG1 fusion proteins, demonstrating potential efficacy against NRG1-positive cancers.
In June 2023, the FDA granted Zenocutuzumab Breakthrough Therapy designation. Subsequently, in May 2024, the FDA accepted its Biologics License Application (BLA) and granted it Priority Review status for the treatment of patients with NRG1 fusion-positive NSCLC and pancreatic ductal adenocarcinoma (PDAC). Dr. Andrew Joe, Chief Medical Officer of Merus, noted that Zeno has the potential to become the first targeted therapy for patients with NRG1-positive lung and pancreatic cancers, offering substantial improvements over currently available therapies.
- Izalontamab
Izalontamab, also known as SI-B001, is a promising first-in-class bispecific antibody targeting EGFR and HER3, developed by Bai Li. This innovative therapy effectively blocks the binding of the EGF to EGFR and the interaction between HER3 and NRG1. By inhibiting these interactions, Izalontamab prevents the formation of both EGFR homodimers and EGFR-HER3 heterodimers, which are crucial for the activation of signaling pathways associated with tumor growth, survival, and metastasis.
Currently, Izalontamab is undergoing six clinical trials targeting various indications, including NSCLC, colorectal cancer, esophageal squamous cell carcinoma, and head and neck squamous cell carcinoma. The drug has shown promising efficacy and good tolerability in several phase 2 clinical trials, with the NSCLC indication progressing to phase 3 trials. It is anticipated that Izalontamab could be approved for use in China between 2024 and 2025. Additionally, the clinical study of SI-B001 in combination with docetaxel has received FDA approval.
3.3 HER3 Monoclonal Antibodies
Currently, there are approximately 24 monoclonal antibodies targeting HER3 worldwide. Among these, 6 are in the preclinical stage, while 4 are in clinical development. The remaining antibodies are either in a state of no further progress or have been terminated. These monoclonal antibodies represent a diverse array of therapeutic strategies aimed at exploiting the unique properties of HER3 in cancer treatment, reflecting the growing interest in this target as a potential avenue for effective therapies.
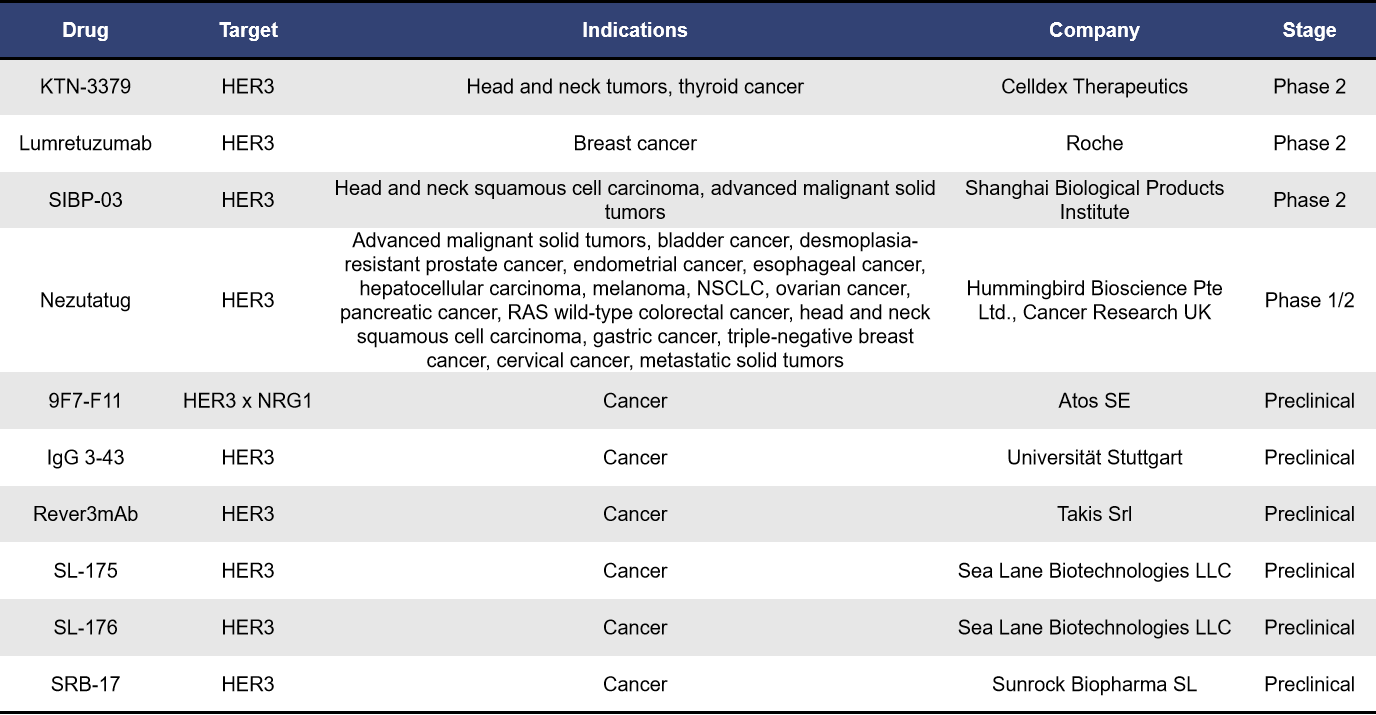
- KTN-3379
KTN-3379, also known as CDX-3379, is a human IgG1λ monoclonal antibody developed by Celldex Therapeutics. It has a high affinity for a unique epitope located between the domains II and III of HER3. By binding to this epitope, KTN-3379 locks HER3 in its inactive conformation, effectively inhibiting both ligand-dependent and ligand-independent activation of HER3. Preclinical studies have shown that the combination of CDX-3379 with cetuximab or BYL719 (a selective PI3Kα inhibitor) can enhance growth inhibition in head and neck squamous cell carcinoma (HNSCC) xenograft models. Currently, KTN-3379 is in clinical phase II trials, highlighting its potential as a promising therapeutic agent in the ongoing battle against cancer.
- Lumretuzumab (RG7116)
Lumretuzumab, also known as RG7116, is a glycoengineered humanized IgG1 monoclonal antibody developed by Roche. It specifically binds with high affinity to domain I of HER3. By blocking the interaction between the ligand heregulin and HER3, lumretuzumab inhibits HER3 dimerization and phosphorylation, ultimately suppressing tumor growth in mouse models based on cell lines. Currently, it is in phase II clinical trials.
- SIBP-03
SIBP-03, developed by the Shanghai Institute of Biological Products, is a targeted HER3 IgG1 monoclonal antibody. It is notable for being the first anti-HER3 antibody drug in China to receive clinical approval. Like lumretuzumab, SIBP-03 is also in phase II clinical trials, indicating its potential in the treatment landscape for cancers associated with HER3.
In addition to the aforementioned biologics, there are various other therapeutic approaches targeting HER3, including small molecules, fusion proteins, vaccines, and CAR-T therapies.
Small Molecules: Among these, AZD-8931, developed by AstraZeneca, is the most advanced, currently in phase II clinical trials. Vaccines: Several vaccine candidates are in the preclinical stage, with companies such as Imugene and Harvard Medical School actively involved in their development. Fusion Proteins: The fusion protein SAL-007, developed by Shenzhen New Horizon, is progressing rapidly and is also in phase II clinical trials.
4. Supporting HER3 Biotherapeutics Development with DIMA Biotechnology
DIMA Biotechnology is a biotechnology company focused on providing druggable target preclinical research products and services. We offer a comprehensive range of products and services targeting HER3. Our product offerings include active proteins, reference antibodies, and flow-validation monoclonal antibodies. Additionally, we provide customized antibody services across multiple species, as well as antibody humanization and affinity maturation services.
To accelerate the development of HER3 biotherapeutics, we have established a HER3 single B cell seed bank, allowing for lead antibody molecules to be generated in as little as 28 days. Currently, we have screened 22 HER3 lead molecules, with 18 showing cross-reactivity in human and monkey proteins. Clients can receive these molecules the next day for functional evaluation. Furthermore, we are actively validating the internalization activity and cytotoxicity of certain molecules for ADC applications, and we welcome inquiries for detailed data.
- HER3 Protein, Antibody, Stable Cell Line, and CDX Slide
- Progress on HER3 Lead mAb Molecules

References:
[1] Maennling AE, Tur MK, Niebert M, et al. Molecular Targeting Therapy against EGFR Family in Breast Cancer: Progress and Future Potentials. Cancers (Basel). 2019;11(12).
[2] Esparís-Ogando A, Montero JC, Arribas J, et al. Targeting the EGF/HER Ligand-Receptor System in Cancer. Curr Pharm Des. 2016;22(39):5887–98.
[3] Kraus MH, Issing W, Miki T, et al. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc Natl Acad Sci U S A. 1989;86(23):9193–7.
[4] Cho HS, Leahy DJ. Structure of the extracellular region of HER3 reveals an interdomain tether. Science. 2002, 297 (5585): 1330–1333.
[5] Roskoski R. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacological Research. 2014, 79: 34–74.
[6] Gandullo-Sánchez, L., Ocaña, A. & Pandiella, A. HER3 in cancer: from the bench to the bedside. J Exp Clin Cancer Res. 2020, 41, 310.
[7] Linggi B, Carpenter G. ErbB receptors: new insights on mechanisms and biology. Trends Cell Biol. 2006;16(12):649–56.
[8] Zhang X, Gureasko J, Shen K, et al. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125(6):1137–49.
[9] Shi F, Telesco SE, Liu Y, et al. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proceedings of the National Academy of Sciences of the United States of America. 2010, 107 (17): 7692–7697.
[10] Gaborit N, Lindzen M, Yarden Y. Emerging anti-cancer antibodies and combination therapies targeting HER3/ERBB3. Human Vaccines & Immunotherapeutics. 2016 Mar;12(3):576-592.