On November 30, 2023, Inipharm, a pharmaceutical company focused on severe liver diseases, announced the initiation of a Phase I clinical study for its oral drug INI-822. INI-822 is the first small molecule inhibitor of HSD17B13 to enter clinical development, and it is also the first clinical candidate developed by Inipharm, primarily intended for the treatment of fibrotic liver diseases, including non-alcoholic steatohepatitis (NASH). What makes HSD17B13 so attractive to Inipharm as its first clinical candidate, and who are the other players in HSD17B13-targeted drugs?
1. Structure and Distribution of HSD17B13
Hydroxysteroid 17β-dehydrogenase 13 (HSD17B13) is an enzyme that belongs to the HSD17B family, which is involved in various aspects of lipid and steroid metabolism. HSD17B13 is also known as SCDR9 or SDR16C3, and it is mainly found in lipid droplets (LDs), which are small organelles that store lipids in cells. HSD17B13 has 15 other members in its family, and it is most closely related to HSD17B11 (85% sequence similarity). Some of the HSD17B enzymes are responsible for activating or inactivating sex hormones, such as HSD17B1, HSD17B2, HSD17B3, HSD17B5, and HSD17B6. Others are involved in fatty acid metabolism, cholesterol biosynthesis, and bile acid production, such as HSD17B4, HSD17B7, HSD17B8, HSD17B9, HSD17B10, HSD17B11, HSD17B12, HSD17B14, and HSD17B15.
The HSD17B13 gene was initially cloned from the human liver cDNA library in 2007 by Liu S et al. and originally named SCDR9 [1]. The human HSD17B13 gene is approximately 17 kb long, located on chromosome 4q22.1, and comprises 8 exons and 7 introns. Through alternative splicing, it can produce 9 different protein isoforms with molecular weights ranging from 22 to 33 kDa. The HSD17B13 protein has two characteristic motifs that are shared by all SCDR family members: one is the TGXGXXXG motif, which binds to NAD(P)H, and the other is the YXXXK motif, which is essential for its catalytic activity (x represents any amino acid residue).
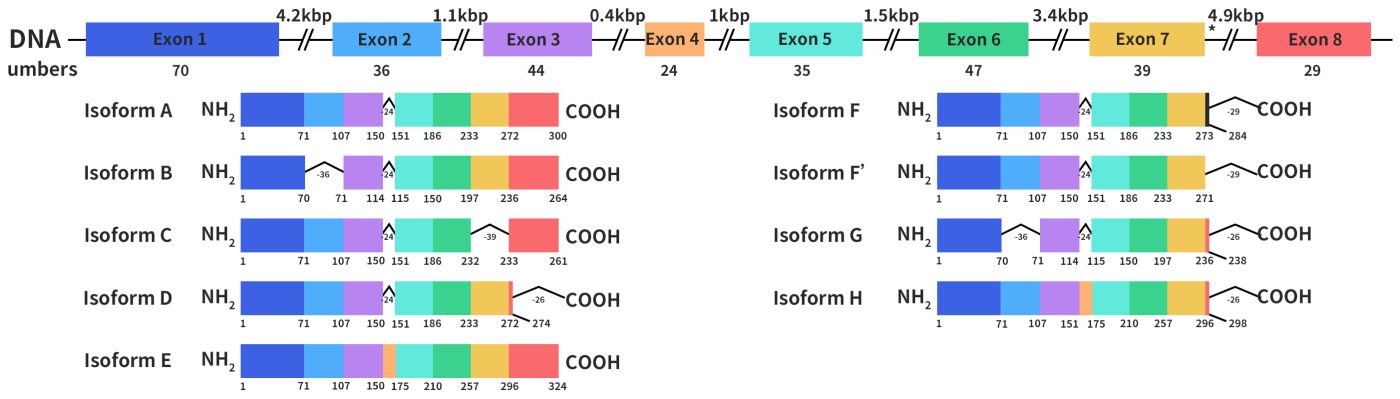
Figure 1. the structures of HSD17B13 gene and protein [1]
Studies have shown that human HSD17B13 is a liver cell-specific LD-associated protein, expressed most abundantly in the liver and at lower levels in the ovaries, bone marrow, kidneys, brain, lungs, skeletal muscles, bladder, and testes. HSD17B13 is mainly located on the membrane of LD in the liver. The first 28 amino acids of its N-terminal sequence are essential for its LD localization [2]. HSD17B13 regulates LD formation, growth, and breakdown in the liver. A genome-wide association study identified a splicing variant (rs72613567:TA) in the HSD17B13 gene, associated with reduced levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in patients with fatty liver, indicating reduced inflammation and liver injury [3].
2. Mechanism of Action of HSD17B13
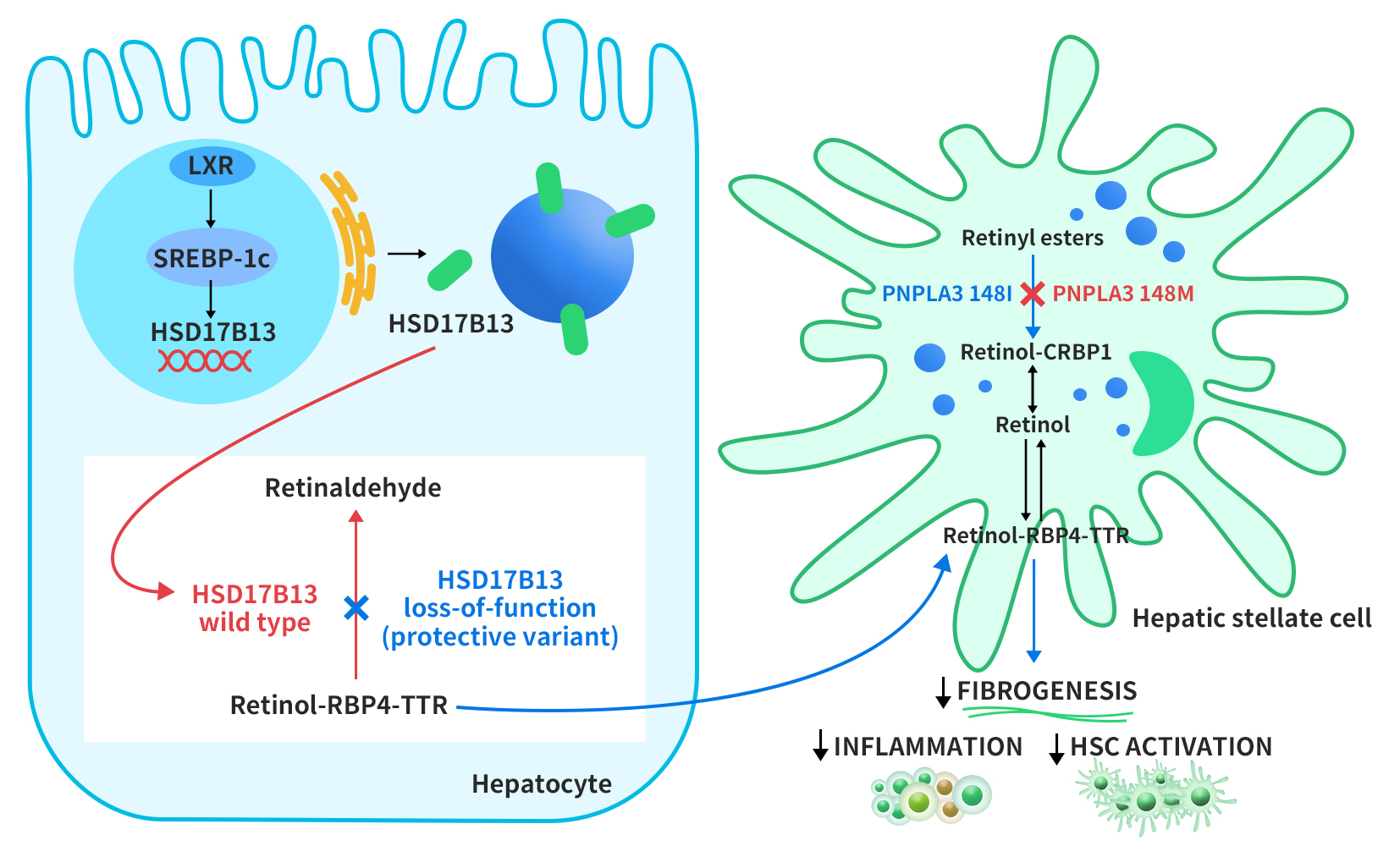
The regulation of HSD17B13 expression is not well understood. However, a study by Su et al. in 2017 suggested that HSD17B13 expression is induced by liver x receptor-α (LXR-α), a nuclear receptor that controls lipid metabolism genes, through the activation of sterol regulatory element-binding protein-1c (SREBP-1c). HSD17B13 then targets lipid droplets (LDs) from the endoplasmic reticulum. HSD17B13 normally converts retinol to retinal, a form of vitamin A. However, some genetic variants of HSD17B13 lose this enzyme activity, and this leads to faster export of retinol-binding protein 4-thyroxine-binding protein (RBP4-TTR) from liver cells. Another LD protein, PNPLA3, breaks down retinyl esters in hepatic stellate cells (HSCs), which are involved in liver fibrosis. A mutation in PNPLA3 (I148M) reduces this activity, and this causes retinol accumulation in HSCs. Vitamin A-like compounds have important effects on liver immunity and fibrosis inhibition by HSCs [4].
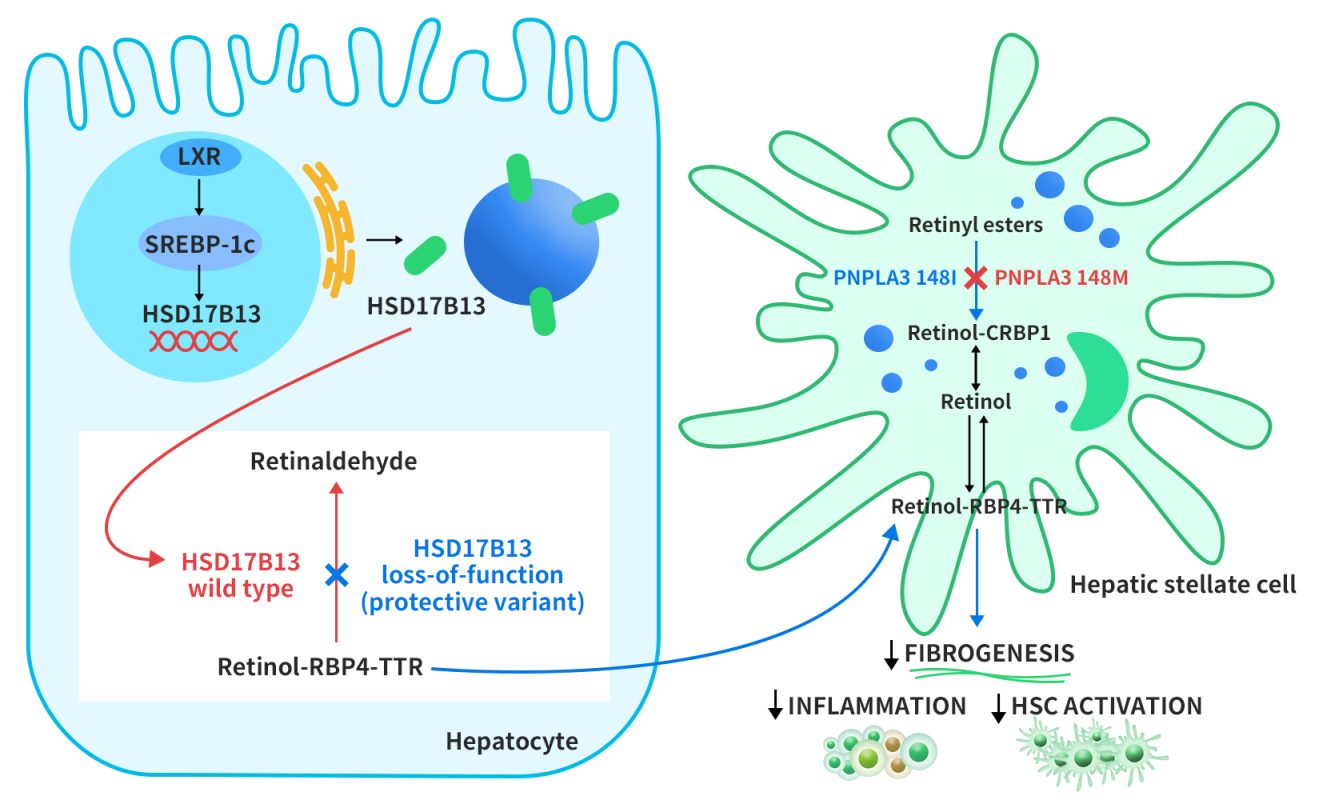
Figure 2. Proposed role of HSD17B13 in modulating disease progression in NAFLD [4]
3. Therapeutic Strategies for Targeting HSD17B13 in NASH
HSD17B13 is an enzyme that regulates lipid metabolism in the liver and is associated with the risk and severity of NAFLD. However, its biological role and molecular mechanisms are not fully understood. Despite this knowledge gap, HSD17B13 has been recognized as a promising target for NAFLD treatment based on human genetics and preclinical studies. Several therapeutic approaches that aim to modulate HSD17B13 activity have entered clinical trials. These include RNA interference (RNAi) therapy and small molecule inhibitors, which are still in the early stages of development.
3.1 RNAi Therapy
RNA interference (RNAi) is a natural cellular process that suppresses gene expression by using double-stranded RNA to inhibit the translation or degrade the mRNA with the same sequence. RNAi can be used to block HSD17B13 expression by introducing synthetic or expressed double-stranded RNA molecules that target the HSD17B13 gene.
ALN-HSD
ALN-HSD is an investigational RNAi therapeutic that targets HSD17B13, a gene associated with NASH. It is developed by Alnylam Pharmaceuticals and Regeneron Pharmaceuticals, and it is a N-acetyl galactosamine (GalNAc)-conjugated siRNA delivered by subcutaneous injection. A Phase I study, presented by Sanyal A et al. at the 2023 EASL Congress, showed that ALN-HSD was safe and well-tolerated in healthy volunteers and NASH patients. ALN-HSD also reduced HSD17B13 mRNA levels in the liver, lowered liver enzymes, and improved NAS scores, which measure the severity of NASH.
ARO-HSD
ARO-HSD, developed by Arrowhead Pharmaceuticals, is also a N-acetyl galactosamine (GalNAc)-conjugated siRNA delivered by subcutaneous injection. ARO-HSD uses double-stranded RNA molecules to silence HSD17B13 mRNA and protein in liver cells. A Phase I/II study, NCT04202354, is testing the safety, tolerability, pharmacokinetics, and pharmacodynamics of ARO-HSD in healthy volunteers and NASH patients. Interim results, presented at the 2021 EASL Congress, showed that ARO-HSD reduced HSD17B13 mRNA and protein levels in the liver, as well as liver enzymes (ALT and AST) in the blood, which indicate liver damage. ARO-HSD is the first drug to achieve this effect on HSD17B13.
In November 2021, Arrowhead and GSK signed an exclusive development license agreement for ARO-HSD, which is now called GSK4532990. GSK has the rights to develop and commercialize the drug in all regions except Greater China, where Arrowhead retains the rights.
3.2 Small Molecule Inhibitors
Due to their small molecular size, small molecule inhibitors can easily penetrate cell membranes to target intracellular proteins. Small molecule inhibitors targeting HSD17B13 have garnered attention in the pharmaceutical industry over the past few years. However, the only one that has entered clinical trials is INI-822. INI-822 is a small molecule inhibitor targeting HSD17B13 developed by Inipharm. It is currently undergoing Phase I trials for the indication of non-alcoholic steatohepatitis (NASH) (NCT05945537). NCT05945537 is a randomized, double-blind, placebo-controlled, single, and multiple dose-escalation study designed to evaluate the safety, tolerability, and pharmacokinetics of the drug in patients with NASH or suspected NASH and in healthy subjects. The trial is currently in the recruitment phase.
In addition to INI-822, Boehringer Ingelheim has also developed a small molecule inhibitor, BI-3231, targeting HSD17B13. BI-3231 is a potent and selective inhibitor of human and mouse HSD17B13, with an activity of 1 nM and a selectivity of 10,000-fold over HSD17B11, a closely related enzyme. However, BI-3231 has not entered clinical trials yet, as it has some drawbacks in its pharmacokinetic properties, such as high clearance, low exposure, and short half-life in vivo.
4. About DIMA Biotechnology
DIMA Biotech is a biotechnology company dedicated to the preclinical research and development of druggable targets. The company’s goal is to prepare pre-validated lead antibody molecules for all druggable targets. This initiative aims to help pharmaceutical companies bypass the challenges of setting up a monoclonal antibody platform and screening lead antibody molecules, and instead focus more on the biological mechanisms and producibility studies of drug targets, thus speeding up the clinical pipeline. DIMA has four main technology platforms:
- DiMPro™ Functional Membrane Protein Development Platform (ECD, Synthetic Nanodisc, VLP);
- DIMA mAbs Single B Cell Lead Antibody Discovery Platform (300+ drug targets completed within 3 years, with the development of 5000+ rabbit monoclonal antibody molecules);
- DiLibrary™ Antibody Engineering and Modification Platform (humanization, affinity maturation, PTM removal, etc.);
- Antibody Functional Validation Platform (CAR-T, ADC antibody functional assessment, etc.).
Reference:
[1] Liu S, Huang C, Li D, Ren W, Zhang H, Qi M, Li X, Yu L. Molecular cloning and expression analysis of a new gene for short-chain dehydrogenase/reductase 9. Acta Biochim Pol. 2007;54(1):213-8. Epub 2007 Feb 20.
[2] Zhang HB, Su W, Xu H, Zhang XY, Guan YF. HSD17B13: A Potential Therapeutic Target for NAFLD. Front Mol Biosci. 2022 Jan 7;8:824776.
[3] Anstee QM, Darlay R, Cockell S, et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort☆. J Hepatol. 2020 Sep;73(3):505-515.
[4] Su W, Peng J, Li S, Dai YB, Wang CJ, Xu H, Gao M, Ruan XZ, Gustafsson JÅ, Guan YF, Zhang XY. Liver X receptor α induces 17β-hydroxysteroid dehydrogenase-13 expression through SREBP-1c. Am J Physiol Endocrinol Metab. 2017 Apr 1;312(4):E357-E367.