On July 16, 2024, Dr. Roger Cone, co-founder of the preclinical biotech company Courage, published a study in The Journal of Clinical Investigation titled “Subthreshold activation of the melanocortin system causes generalized sensitization to anorectic agents in mice.” The research demonstrated the synergistic effect between GLP-1 receptor agonists (GLP1RA) and MC4R agonists, revealing that low doses of MC4R agonists can significantly enhance the dose responsiveness of GLP1RA drugs. This leads to reduced food intake and promotes weight loss through a novel mechanism that improves the efficacy of GLP1RA drugs without increasing side effects. But what exactly is MC4R, and what role does it play in the weight loss process?
1. MC4R and the Melanocortin Receptor Family
The melanocortin 4 receptor (MC4R) is a member of the melanocortin receptor family, which also includes MC1R, MC2R, MC3R, and MC5R. This family is a subfamily of the GPCR superfamily, specifically the rhodopsin-like class A GPCRs, and holds significant therapeutic potential. Melanocortin receptors (MC1R-MC5R) are found on various cell types and are distributed throughout most body systems. MC1R and MC3R are located on immune cells and related structural and supporting cells, making them key receptors that directly influence the immune system. Upon activation by binding with ligands, both MC1R and MC3R can provide direct anti-inflammatory effects and stimulate pro-resolving actions. MC4R, primarily expressed in the central nervous system, plays a crucial role in the central regulation of metabolism, including food intake. MC5R is found in exocrine glands and is expressed by certain immune-active cell subtypes in the eye. MC2R is predominantly expressed in the adrenal glands, where its stimulation is directly linked to the release of cortisol.
2. Structure and Function of MC4R
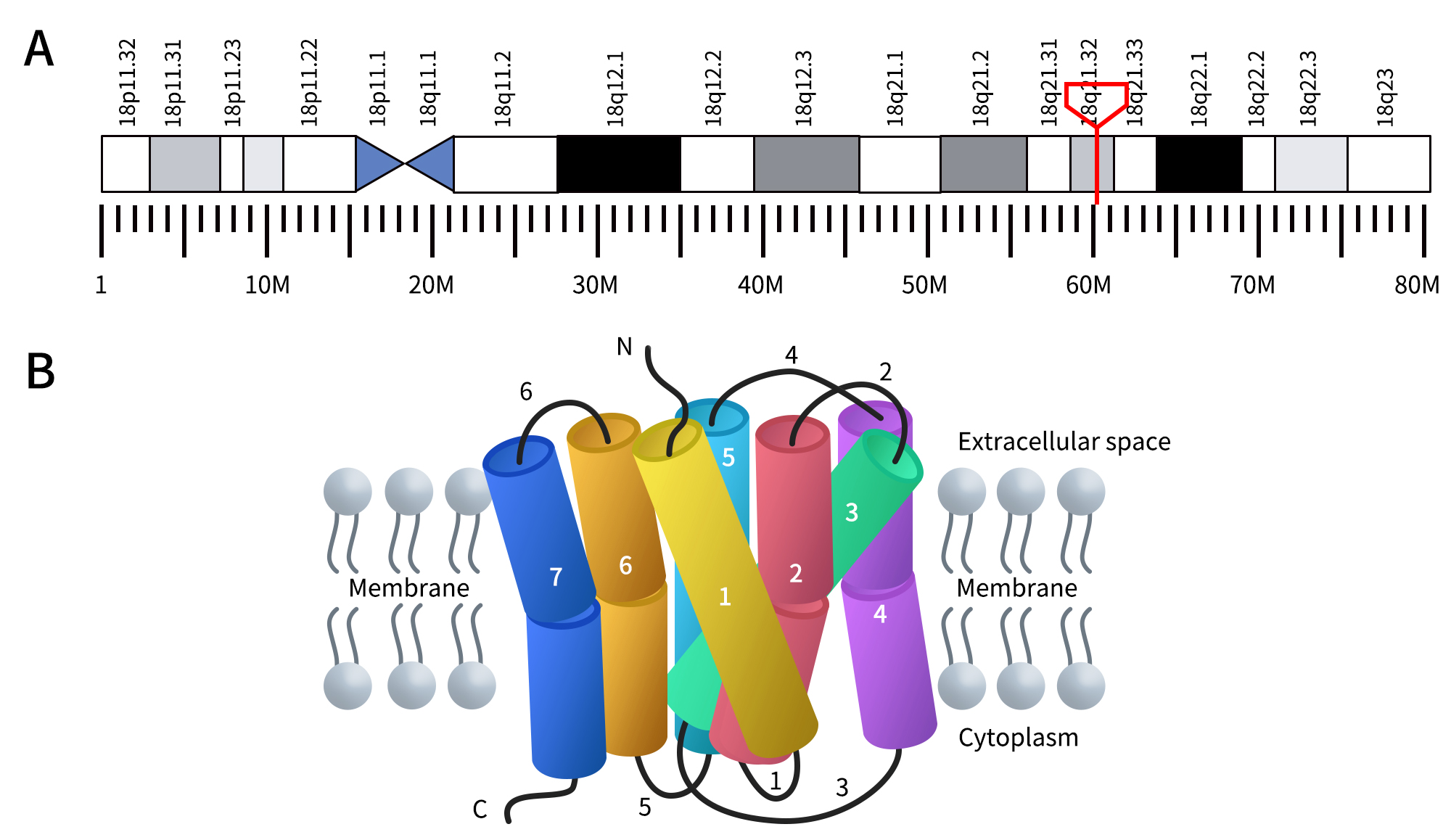
In humans, the melanocortin 4 receptor (MC4R) is encoded by the MC4R gene located at 18q21.32. The MC4R protein is a G protein-coupled receptor (GPCR) that binds to the α-melanocyte-stimulating hormone (α-MSH). Like other members of the GPCR family, the MC4R protein consists of seven transmembrane domains. These transmembrane regions predicted to range between 21 and 27 amino acids in length, are connected by three extracellular loops and three intracellular loops, with an extracellular N-terminus and an intracellular C-terminus. The extracellular loops (loops 2, 4, and 6) vary in length between 5 and 17 amino acids, while the intracellular loops (loops 1, 3, and 6) range between 12 and 33 amino acids in length [1].
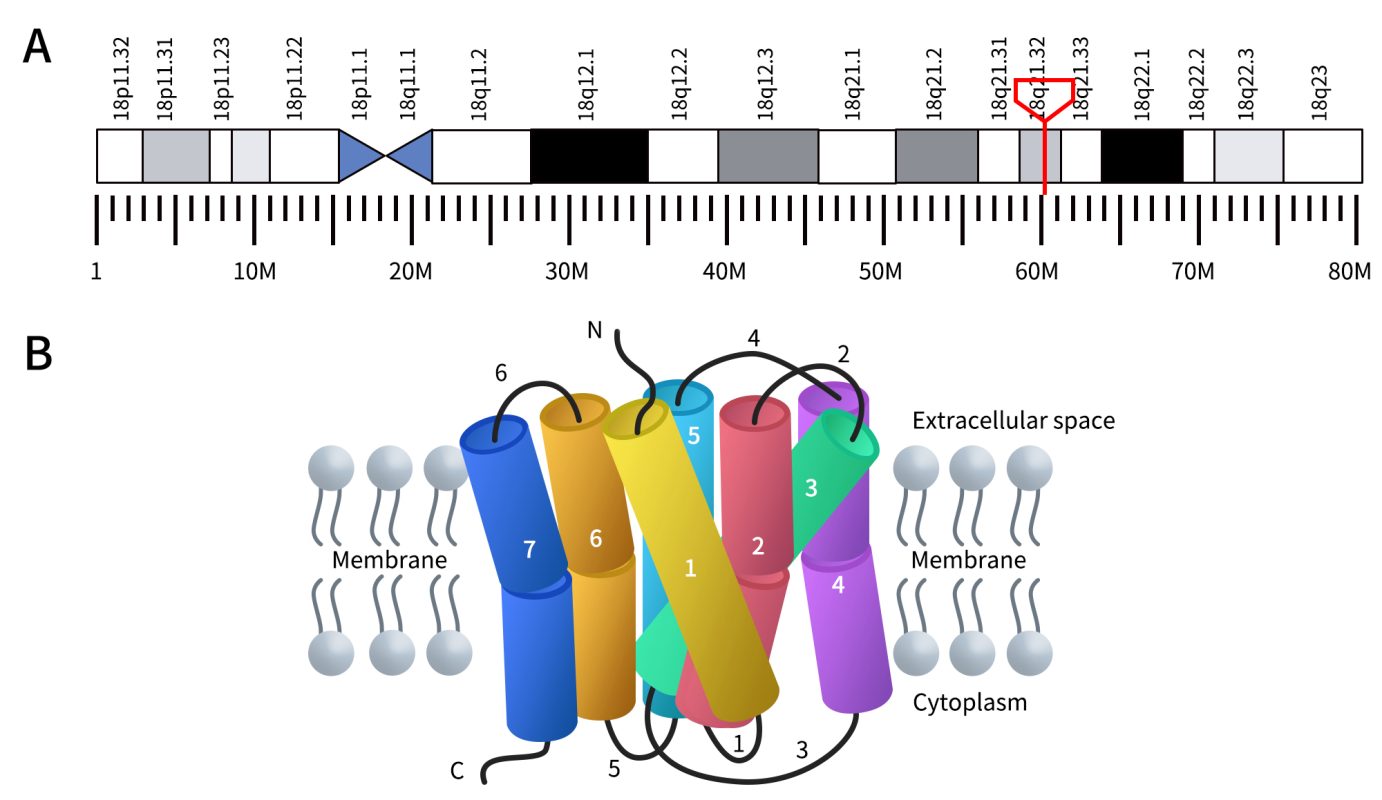
Figure 1. The Location of MC4R gene (A) and the structure of MC4R protein (B) [1] (The gene location is derived from WIKIPWDIA)
As mentioned, MC4R is primarily expressed in the central nervous system and is a receptor found on neurons in the brain that regulate food intake and energy balance. It plays a crucial role in appetite control, eating behavior, and body weight regulation. Activation of MC4R in the brain typically leads to a reduction in food intake and an increase in energy expenditure. Conversely, inhibition of MC4R results in increased food intake and decreased energy expenditure.
3. Melanocortin-4 Receptor (MC4R) Signaling Pathways
MC4R is involved in several key signaling pathways, including the leptin-melanocortin pathway, G protein signaling pathway, β-arrestin pathway, and Ca2+ regulation pathways. These signaling pathways are all closely associated with obesity and energy metabolism.
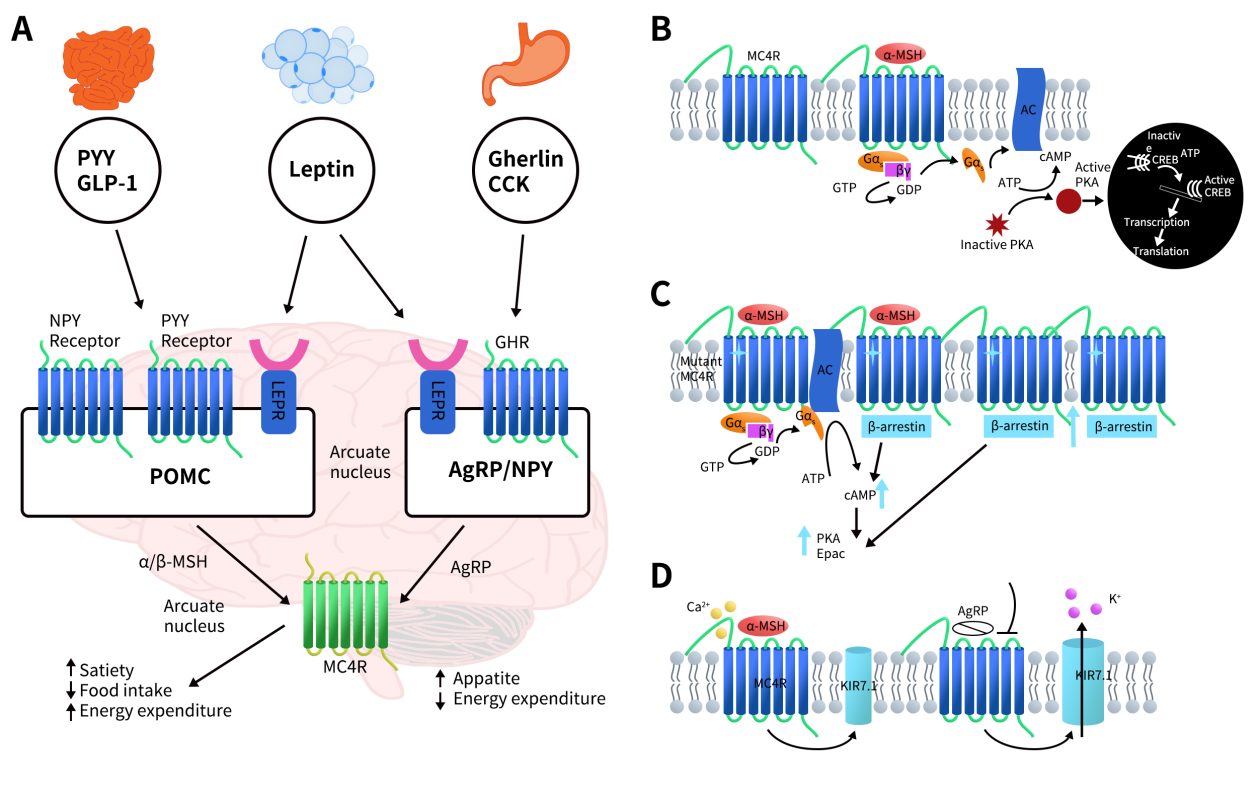
Figure 2. MC4R signaling pathways (A: Leptin melanocortin pathway [2]; B: G-protein signaling pathway[3]; C: β-arrestin signaling pathway[3]; D: Ca2+ regulated pathway[3])
3.1 Leptin-Melanocortin Pathway
In a state of satiety, adipose tissue secretes leptin, which activates leptin receptors (LEPR) in the arcuate nucleus of the hypothalamus. Upon binding to LEPR, leptin stimulates the release of α-melanocyte-stimulating hormone (α-MSH) from pro-opiomelanocortin (POMC) neurons. α-MSH then binds to and activates MC4R located in the paraventricular nucleus (PVN) of the hypothalamus. This activation generates signals of satiety, reducing appetite and increasing energy expenditure. Simultaneously, MC4R activation inhibits the secretion of Agouti-related peptide (AgRP), an antagonist of MC4R produced by neuropeptide Y (NPY) neurons in the arcuate nucleus. In the absence of food, NPY/AgRP expression increases, which generates hunger signals and promotes increased food intake [2].
3.2 G-Protein Signaling Pathway
The classical Gs pathway and the generation of cyclic adenosine monophosphate (cAMP) have been extensively studied in relation to obesity and energy balance, and their mechanisms are well understood. G-proteins are complex heterotrimeric guanine nucleotide-binding proteins composed of Gα, Gβ, and Gγ subunits. When α-MSH binds to MC4R, it activates the G-protein, causing the Gαβγ subunits to dissociate into Gα and Gβγ subunits. The dissociated Gαs subunit activates adenylyl cyclase (AC), which then converts ATP into cAMP. Elevated levels of cAMP further activate protein kinase A (PKA), which translocates to the nucleus. There, PKA phosphorylates the cAMP response element-binding protein (CREB), a transcription factor, thus regulating gene transcription [3].
3.3 β-ARRESTIN Pathway
The β-arrestin pathway has recently been implicated in the regulation of MC4R, particularly in relation to MC4R mutations that cause either loss of function or gain of function (GoF). Mutations leading to GoF may exploit β-arrestin signaling, especially those mutations associated with reduced risk of obesity-related diseases. Studies have shown that MC4R mutations, such as V103I and I251L, facilitate the recruitment of β-arrestin. When mutated MC4R binds to its agonists (e.g., α-MSH or α-MSH analogs), it can induce conformational changes in the receptor, affecting the interaction between the receptor and its co-receptors. This effect might be due to either enhanced binding with co-receptors that prevents MC4R from internalizing into the cell or increased receptor recycling, resulting in prolonged retention of MC4R on the plasma membrane. Consequently, this can lead to elevated levels of intracellular cAMP, protein kinase A (PKA), and cAMP-regulated guanine nucleotide exchange factor (Epac) [4][5].
3.4 Ca2+ Regulation Pathway
Ca2+ serve as cofactors in the binding of ligands to MC4R. Ca2+ can activate MC4R in conjunction with α-MSH, which induces the closure of inward-rectifying potassium channels (KIR7.1). This closure helps maintain intracellular potassium levels, resulting in an overall anorectic effect, characterized by increased satiety and reduced food intake. Conversely, AgRP can open KIR7.1, allowing potassium ions to flow out of the cell, thereby promoting appetite [6][7][8].
4. Clinical Research Progress on MC4R-Targeted Drugs
Globally, there are currently 32 drugs targeting MC4R under development. Among these, 2 have been approved for market, 4 are in clinical stages, 12 are in preclinical stages, 7 have been discontinued, and 7 are in a state of no progress. The drugs encompass a range of types, including small molecule drugs, synthetic peptides, biologics, and CAR-T therapies. Small molecule drugs represent the largest proportion, followed by synthetic peptides.
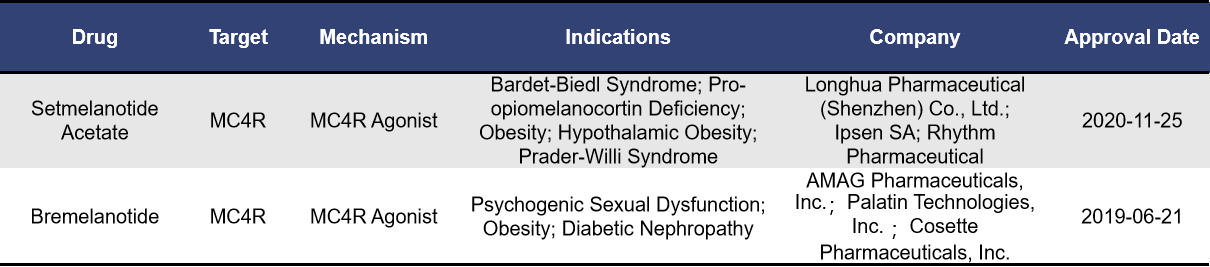
4.1 Approved MC4R-Targeted Drugs
Currently, two MC4R-targeted drugs have been approved and are available on the market, both of which are synthetic peptides. Setmelanotide Acetate, developed by Rhythm Pharmaceuticals, was approved at the end of 2020. Setmelanotide is a single-target MC4R agonist and was previously designated as a breakthrough therapy by the FDA for rare obesity-related conditions. It is approved for the treatment of Alström syndrome, cachexia, leptin deficiency, diabetes, obesity, and other genetic disorders. Bremelanotide, developed by Palatin Technologies, was approved in 2019 for the treatment of premenopausal women with generalized hypoactive sexual desire disorder (HSDD). Bremelanotide acts as an MC4R agonist and has non-selective activation effects on various receptor subtypes. Its activity decreases in the following order: MC1R > MC4R > MC3R > MC5R > MC2R. Within the therapeutic dose range, Bremelanotide’s binding affinity for MC1R and MC4R increases proportionally with dose.
4.2 MC4R-Targeted Drugs in Clinical Trials
Currently, there are 4 MC4R-targeted drugs in clinical development, including LB54640, RM-718, PF-07258669 and TCMCB-07.
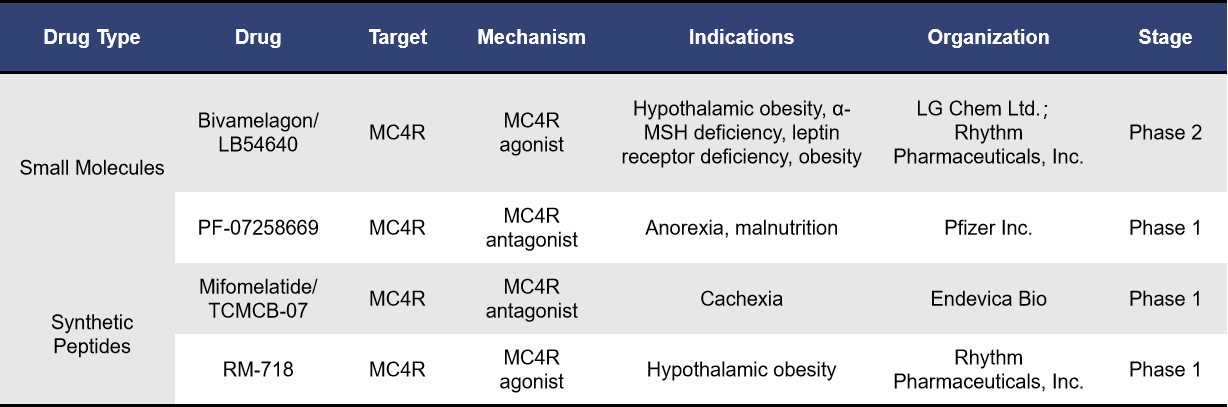
LB54640, developed independently by LG Chem, is an oral MC4R agonist designed primarily for the treatment of rare genetic obesity. In January 2024, LG Chem announced the transfer of global development and commercialization rights for LB54640 to Rhythm Pharmaceuticals. The deal includes an upfront payment of $100 million, with the total contract value reaching $305 million.
Currently, LB54640 is undergoing Phase 2 clinical trials for hypothalamic obesity (NCT06046443). This study aims to recruit 28 patients with congenital or acquired hypothalamic dysfunction who have difficulty controlling appetite and are aged 12 years and older. The trial will assess changes in Body Mass Index (BMI) at 14 weeks of medication use, as well as the safety of long-term use over 52 weeks. The trial commenced on July 11, 2024, and is expected to conclude by early 2026. In addition to LB54640, Rhythm Pharmaceuticals is also advancing the RM-718 project, a more selective MC4R agonist, in clinical trials (NCT06239116).
PF-07258669, developed by Pfizer, is a potent and selective MC4R antagonist. It has demonstrated significant efficacy in elderly rat models of cachexia. Pfizer is conducting Phase 1 clinical trials of PF-07258669 in both the United States (NCT04628793) and Belgium (NCT05113940). These trials aim to evaluate the safety and pharmacokinetics of the drug.
TCMCB-07, developed by Endevica Bio, is a candidate peptide designed to treat cachexia. It acts as an MC3R/MC4R antagonist and has the unique capability to cross the blood-brain barrier, targeting receptors previously inaccessible to therapeutic agents. This allows for modulation of the body’s behavioral and metabolic responses to chronic diseases.
In November 2022, Endevica Bio reported preliminary positive results from the Phase 1 Single Ascending Dose (SAD) trial for TCMCB-07 in the treatment of cachexia. This study focused on evaluating the safety, tolerability, and pharmacokinetics of TCMCB-07 in healthy volunteers. The ongoing Multiple Ascending Dose (MAD) trial is expected to complete by the end of December 2022.
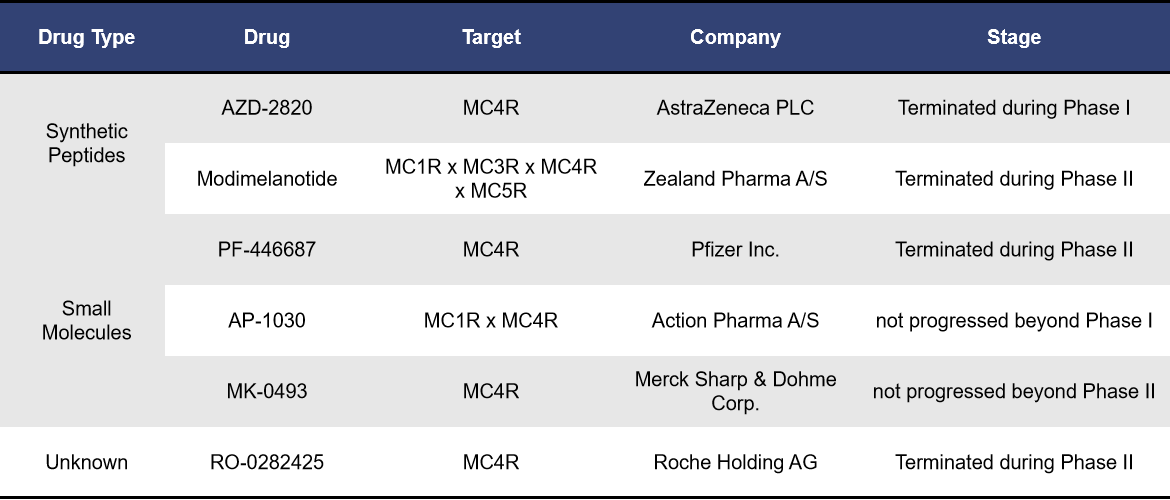
4.3 Clinical Trials with No Progress & Terminated Targeting MC4R
Currently, there are 4 MC4R-targeted drugs that have been terminated during clinical stages and 2 drugs that have shown no progress. Among of them, AZD-2820 (AstraZeneca) was discontinued in 2012 due to severe adverse reactions observed during Phase I clinical trials.
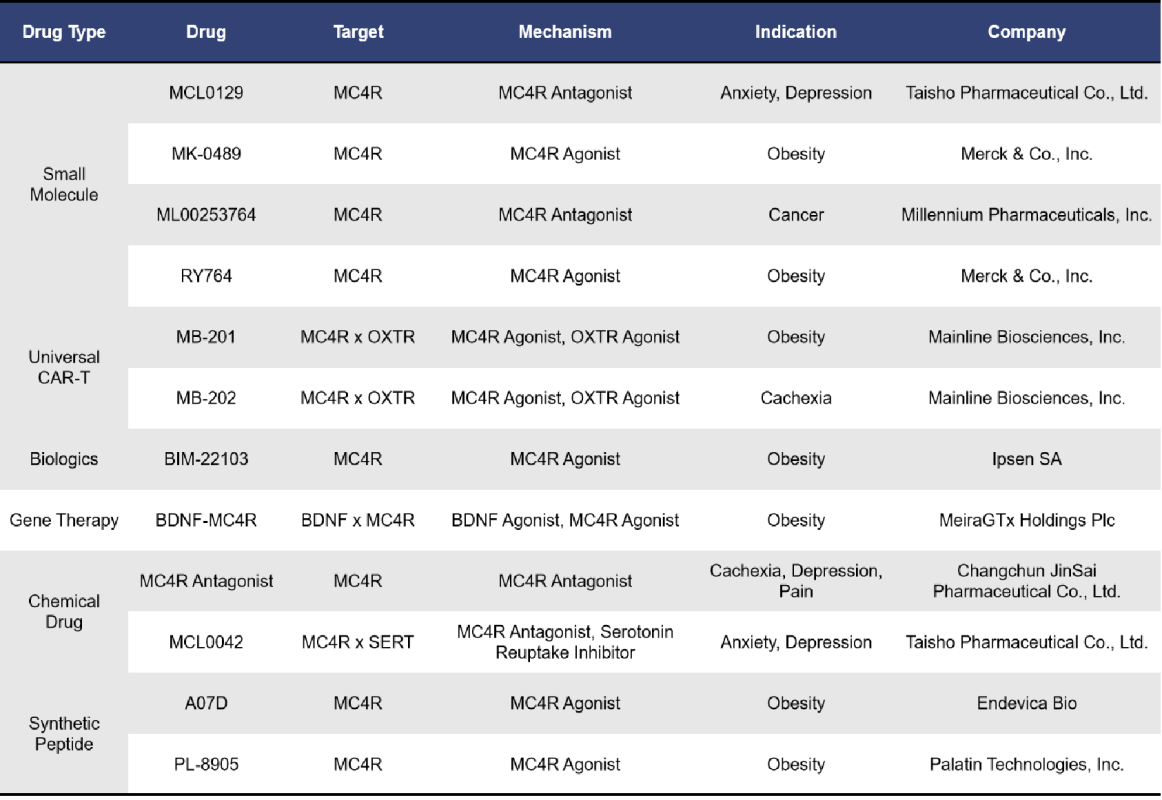
In addition to the drugs in clinical stages, there are 12 MC4R-targeting drugs currently in preclinical development. The details are as follows:
5. DIMA Biotech’s Full-Length MC4R Active Protein Supports Drug Development
DIMA Biotechnology is a biotechnology company focused on preclinical research products and services for druggable targets. Leveraging its proprietary Synthetic Nanodisc membrane protein expression platform, DIMA has successfully developed a full-length human MC4R protein.
Unlike most MSP (Membrane Scaffold Protein) Nanodiscs available in the market, DIMA’s Synthetic Nanodisc is based on an eukaryotic expression system, allowing the production directly from intact cells. In this process, synthetic polymers with dual functions are used. First, they dissolve the cell membrane, similar to detergents, and then utilize the natural cell phospholipids to form the nanodisc structure around the membrane proteins. Transmembrane proteins can be integrated into these Nanodiscs.
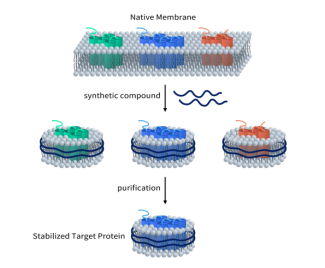
Currently, DIMA has developed hundreds of membrane proteins using the Nanodisc platform. You can click here to view all available membrane proteins. This innovative platform has significant implications for drug development, particularly for complex targets like MC4R, facilitating the production of high-quality active proteins that are essential for preclinical research and screening in drug discovery.
Human MC4R full-length protein-synthetic nanodisc (FLP100122)
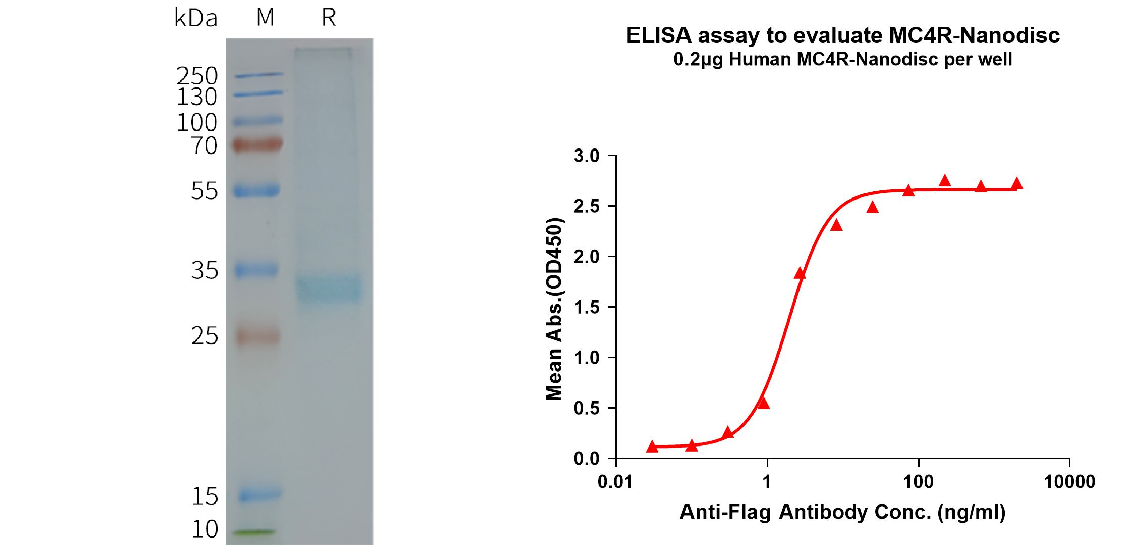
[left] Human MC4R-Nanodisc, Flag Tag on SDS-PAGE.
[Right] ELISA plates were pre-coated with Flag Tag MC4R-Nanodisc (0.2μg/per well). The EC50 for anti-Flag monoclonal antibody binding with MC4R-Nanodisc is 1.957ng/ml.
In addition, DIMA Biotech offers various species protein and antibody customization services, antibody humanization and affinity maturation services, and lead molecule discovery services based on their single B cell antibody discovery platform, functional membrane protein development, and antibody engineering and functional validation platform. For more details, please feel free to contact us.
Reference:
[1]Neocleous V, Shammas C, Phelan MM, et al. A novel MC4R deletion coexisting with FTO and MC1R gene variants, causes severe early onset obesity. Hormones (Athens). 2016 Jul;15(3):445-452.
[2]Chermon, D.; Birk, R. Predisposition of the Common MC4R rs17782313 Female Carriers to Elevated Obesity and Interaction with Eating Habits. Genes 2023, 14, 1996.
[3]Fatima MT, Ahmed I, Fakhro KA, et al. Melanocortin-4 receptor complexity in energy homeostasis,obesity and drug development strategies. Diabetes Obes Metab. 2022 Apr;24(4):583-598.
[4]Brouwers B, de Oliveira EM, Marti-Solano M, et al. Human MC4R variants affect endocytosis, trafficking and dimerization revealing multiple cellular mechanisms involved in weight regulation. Cell Rep. 2021; 34(12):108862.
[5]Lotta LA, Mokrosiński J, Mendes de Oliveira E, et al. Human gain-of-function MC4R variants show signaling bias and protect against obesity. Cell. 2019; 177(3): 597-607.e9.
[6]Yu J, Gimenez LE, Hernandez CC, et al. Determination of the melanocortin-4 receptor structure identifies Ca2+ as a cofactor for ligand binding. Science. 2020; 368(6489): 428-433.
[7]Chaturvedi M, Shukla AK. Calcium as a biased cofactor. Science. 2020; 368(6489): 369-370.
Ghamari-Langroudi M, Digby GJ, Sebag JA, et al. G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature. 2015; 520(7545): 94-98.