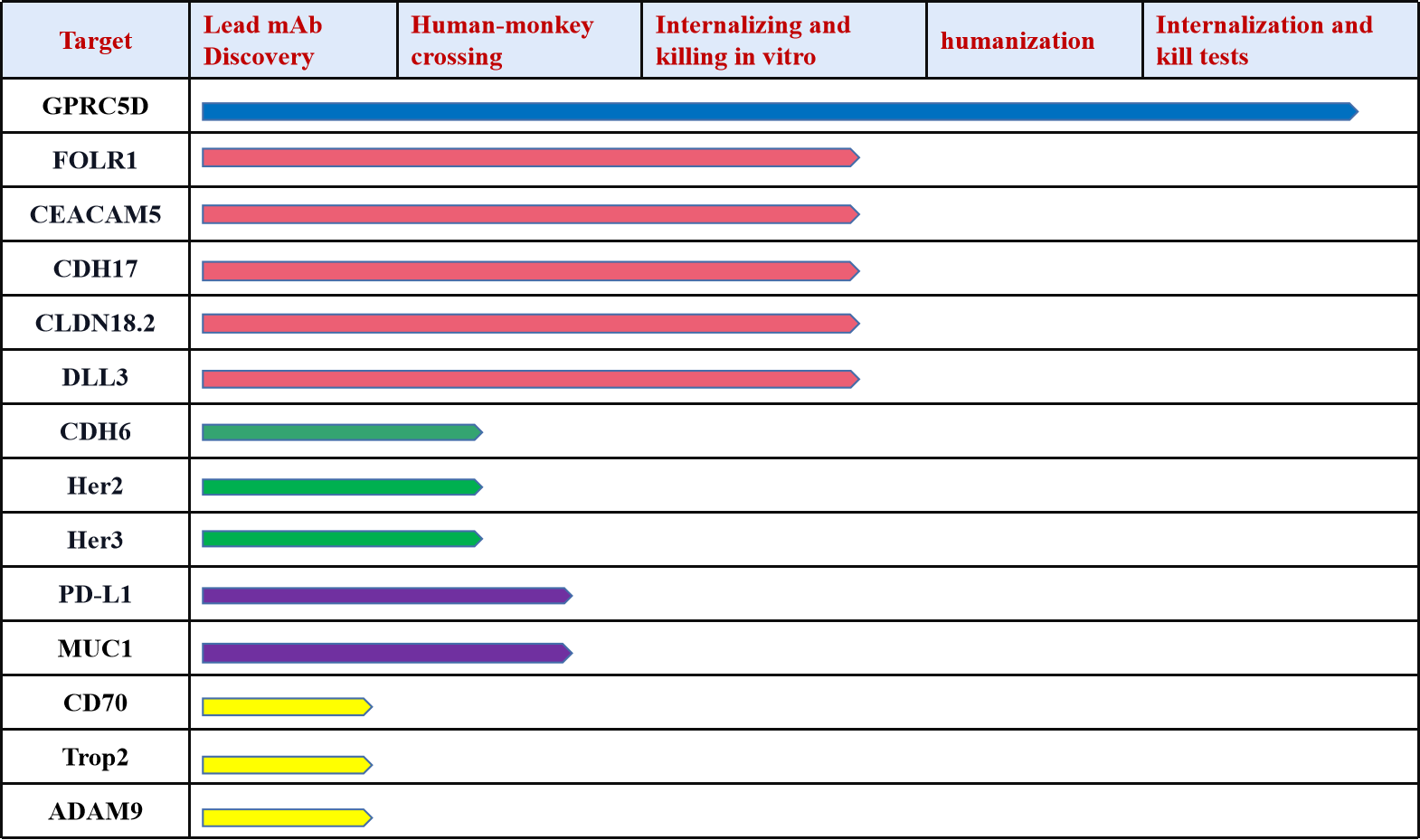
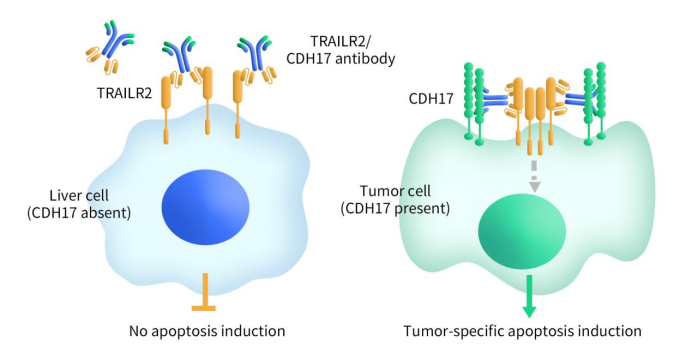
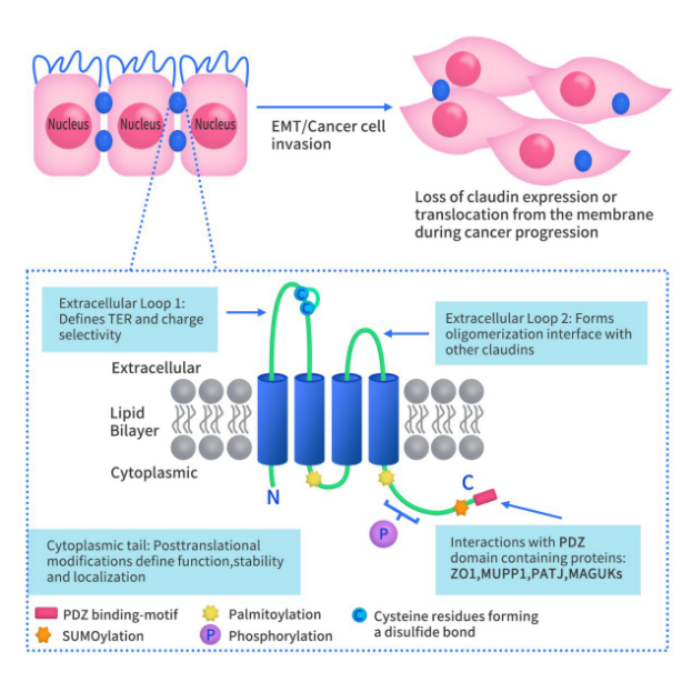
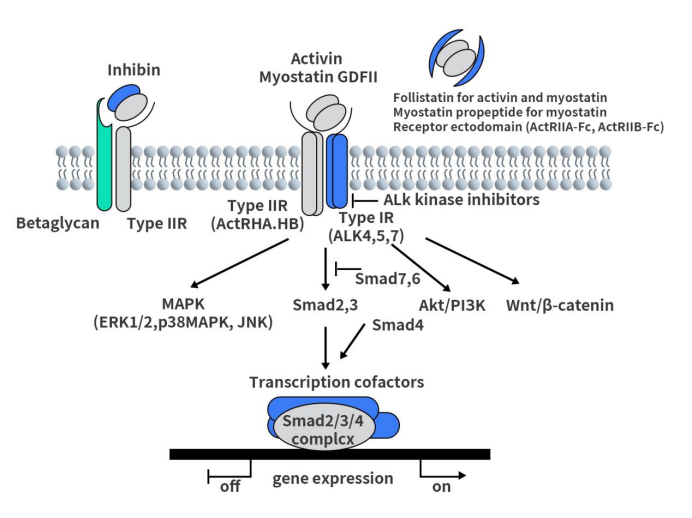
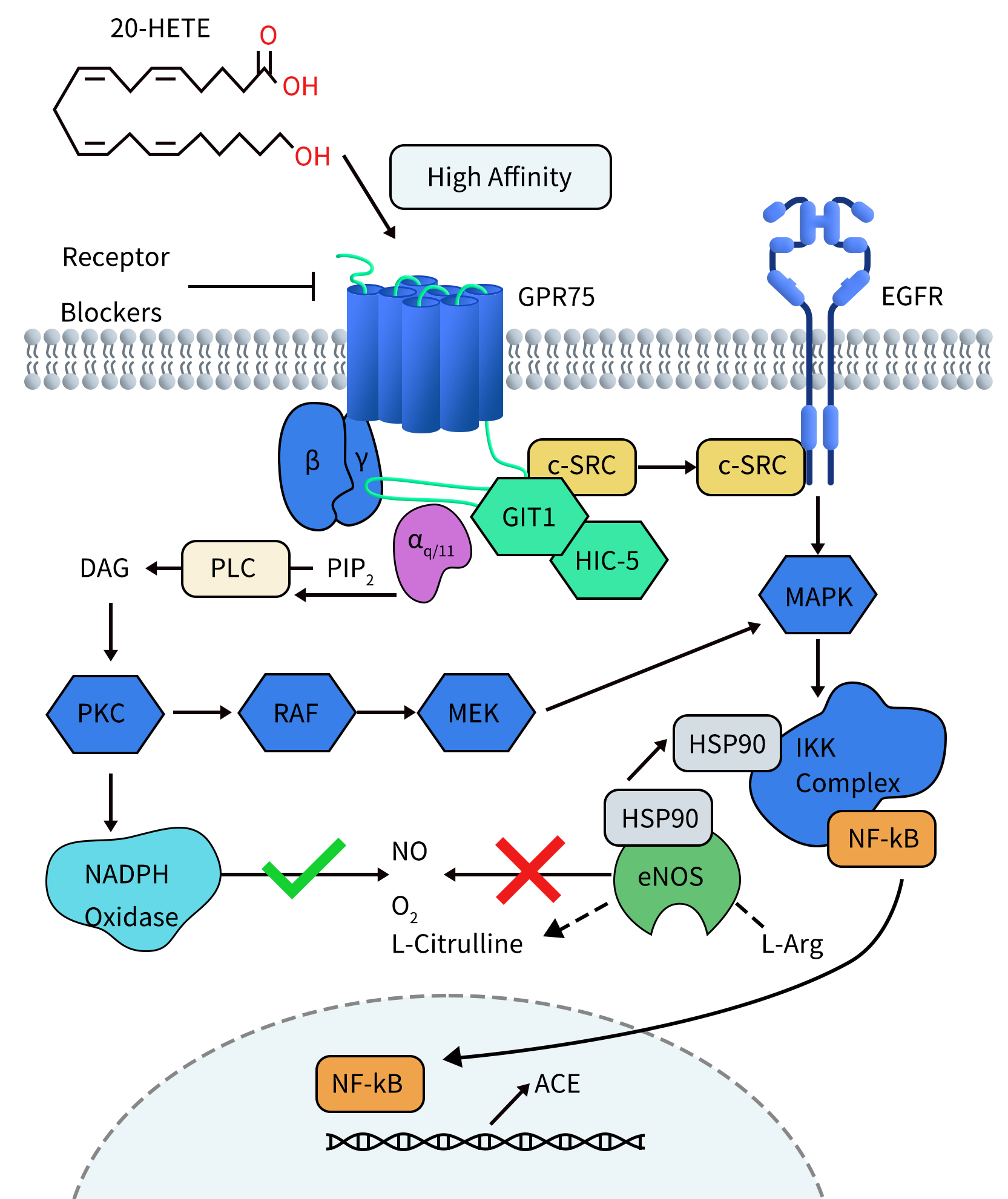
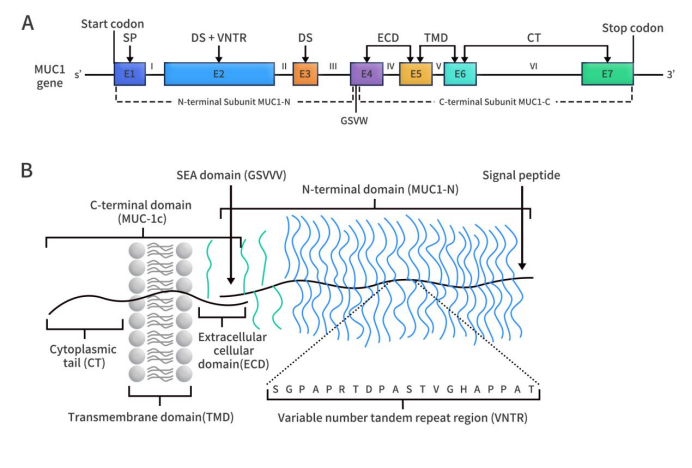
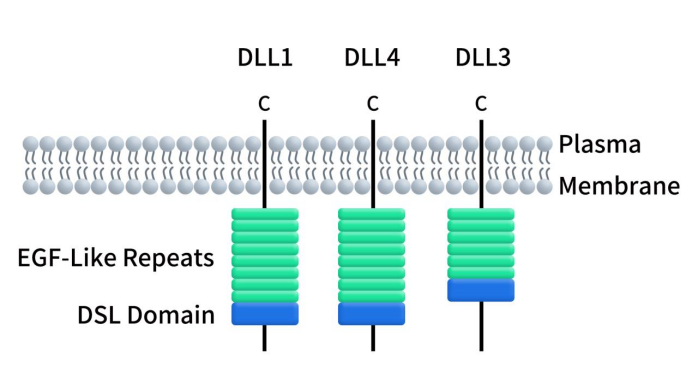
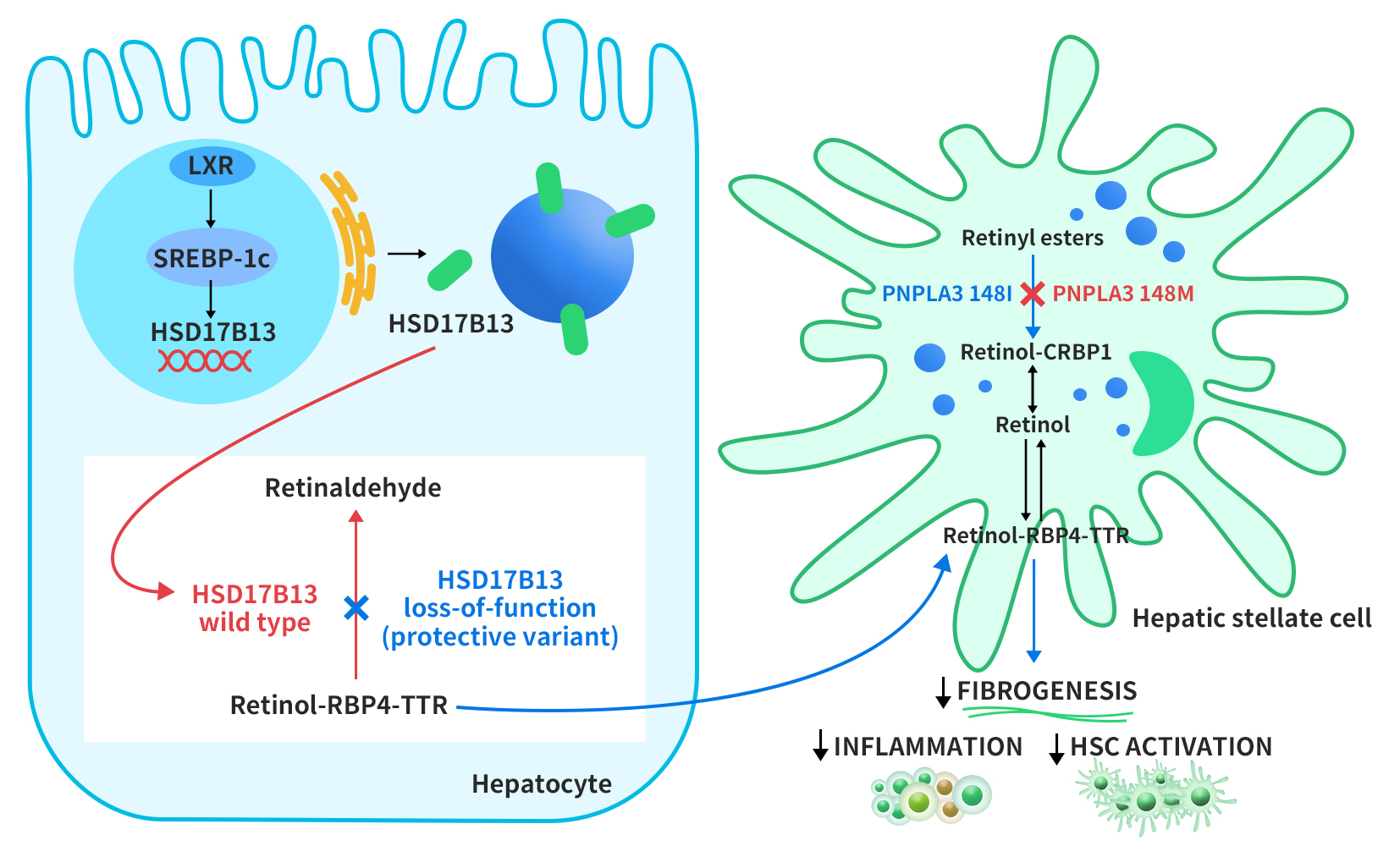
Connect with DIMA Biotechnology this May at Boston
2024 PEGS Time & Location: May 13-17, 2024, Boston Conference Background: PEGS (Essential Protein & Antibody Engineering Summit) is a premier event in the field of protein engineering and biotherapeutics, featuring comprehensive programming covering all aspects of biologic drug development. DIMA Highlights: At this year’s PEGS Summit, DIMA will present a poster describing the innovative […]