Prostate-specific membrane antigen (PSMA) is a pivotal molecular target in the treatment and diagnosis of prostate cancer (PCa). Over the past two decades, extensive research has illuminated its unique molecular characteristics, paving the way for advanced diagnostic and therapeutic approaches. PSMA-targeted imaging techniques, particularly positron emission tomography (PET) radiotracers, have demonstrated exceptional sensitivity in detecting PCa lesions, significantly enhancing detection rates compared to traditional methods. This article delves into the latest advancements in PSMA-targeted radiotherapy and diagnostic treatments, highlighting the transformative impact on prostate cancer management.
1. Structure and Distribution of PSMA
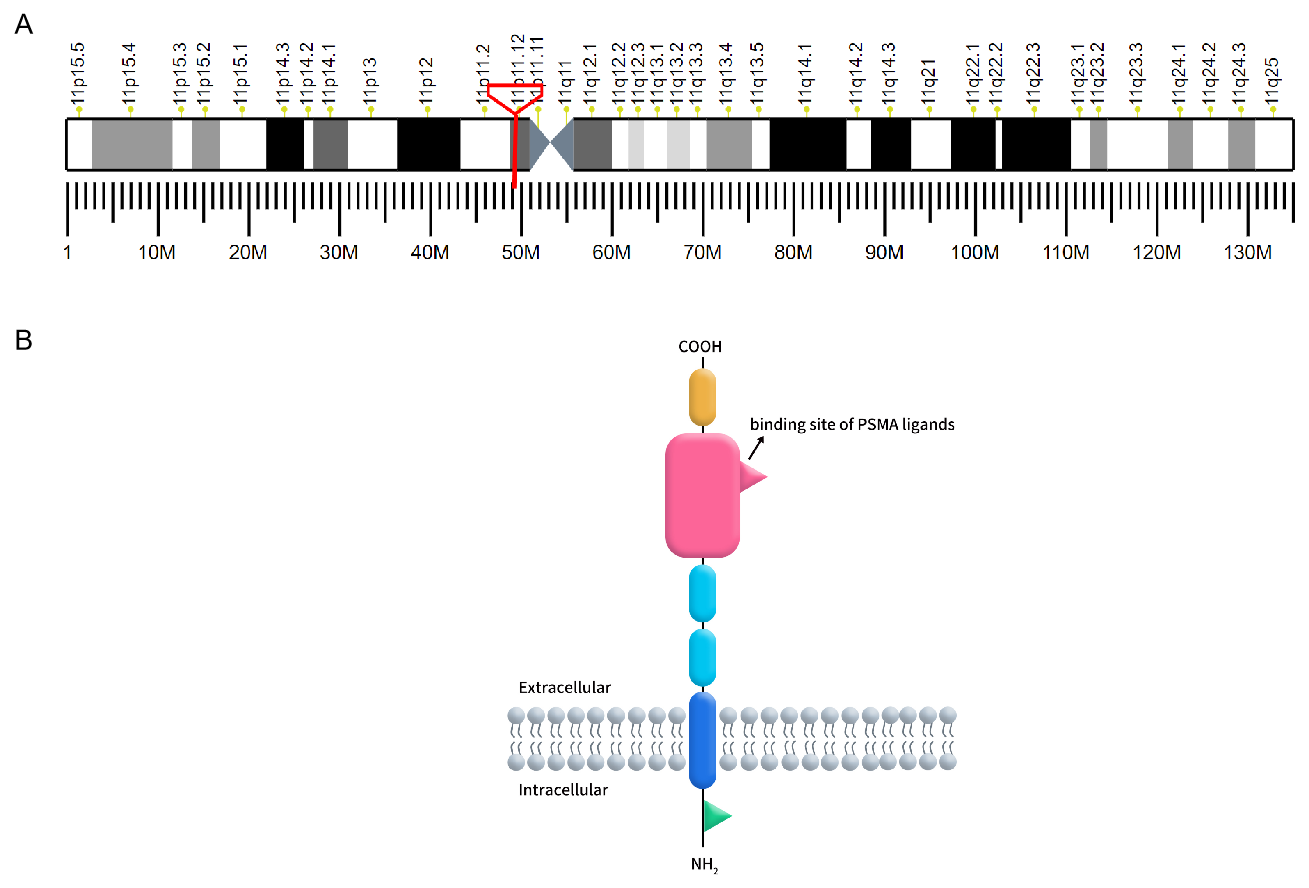
Prostate-specific membrane antigen (PSMA), also known as folate hydrolase 1 or glutamate carboxypeptidase II (GCP II), is a type II transmembrane glycoprotein encoded by the FOLH1 gene located on chromosome 11p11.2. PSMA is characterized by its distinctive three-part structure: a 19-amino acid intracellular domain, a 24-amino acid transmembrane domain, and a 707-amino acid extracellular domain, which includes a ligand-binding site crucial for its function [1]. PSMA is predominantly expressed in prostate epithelial cells but is also found in the neovasculature of various solid tumors, making it a significant target for cancer diagnostics and therapy.
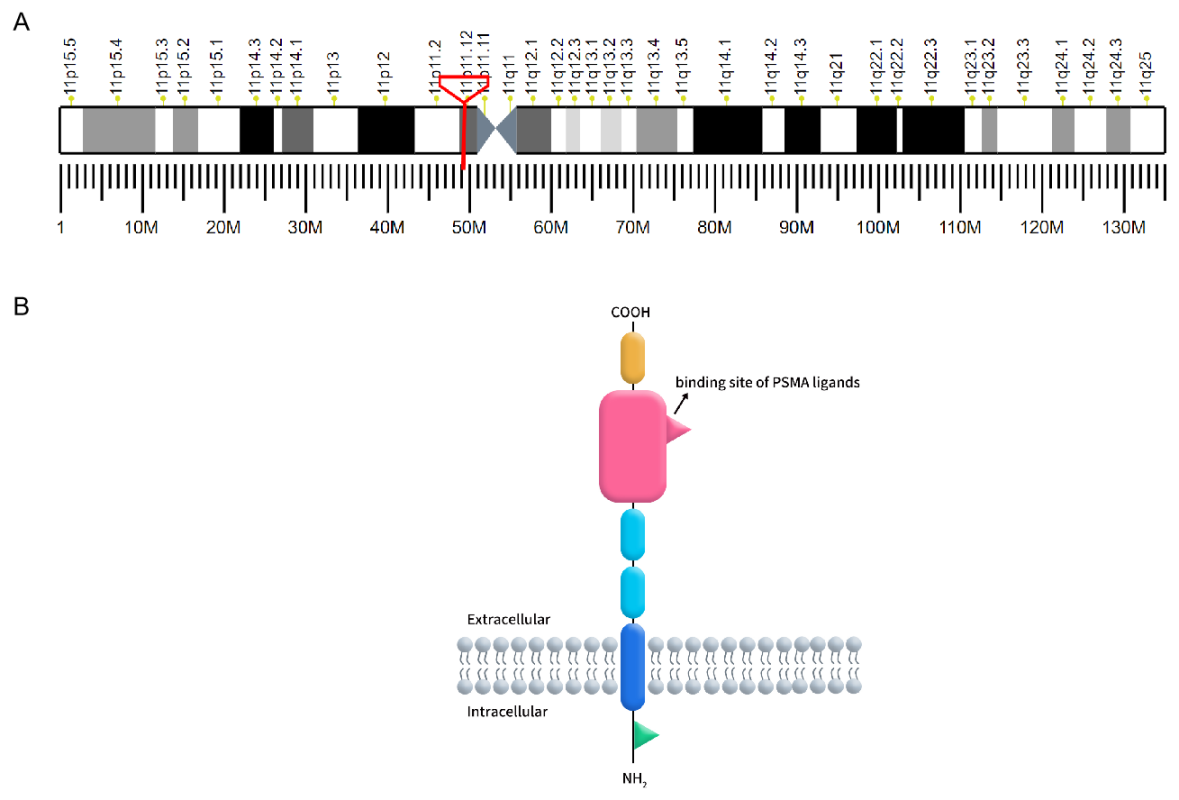
Figure 1. A: The localization of PSMA on human chromosomes; B: The structure of PSMA.
(Cited from Current role of prostate-specific membrane antigen-based imaging and radioligand therapy in castration-resistant prostate cancer. Front Cell Dev Biol, 2022.)
In benign prostate cells, PSMA is located in the cytoplasm and on the apical surface of prostate epithelial cells. Upon malignant transformation, PSMA translocates from the cytoplasm to the surface of the prostate glandular lumen, where it exposes a large extracellular domain to ligands. Additionally, PSMA is expressed in various benign and malignant tissues. Histopathological studies have confirmed PSMA expression in neuroendocrine cells of the salivary glands, duodenal mucosa, proximal renal tubule cells, and colonic crypts. However, the expression levels of PSMA in these tissues are significantly lower than in prostate cancer (PCa) lesions [2].
2.PSMA and the Diagnosis and Treatment of PCa
Studies have shown that, compared to benign prostate tissue, the expression level of PSMA in prostate cancer (PCa) is increased by 100 to 1000 times. Although our understanding of the heterogeneity of PSMA expression between patients and within patients is continually improving, it is generally observed that PSMA expression tends to increase with the degree of tumor dedifferentiation and the progression to metastatic castration-resistant prostate cancer (mCRPC). However, in neuroendocrine prostate cancer, the PSMA gene (FOLH1) may be suppressed, leading to lower PSMA expression levels.
In the diagnosis of prostate cancer (PCa), traditional imaging techniques have limited sensitivity for detecting low-volume PCa lesions, making them less effective in identifying early biochemical recurrent prostate cancer (BRPC) and occult metastatic PCa. For instance, when prostate-specific antigen (PSA) levels are below 0.4 ng/mL, multiparametric magnetic resonance imaging (MRI) shows a low detection rate. Similarly, when PSA levels are suitable for salvage therapy, computed tomography (CT) and bone scans rarely detect the anatomical location of recurrence. Consequently, PSMA-targeted imaging has emerged as a highly appealing alternative. PSMA PET imaging is now integrated into international guidelines for PCa diagnosis and has received approval from regulatory agencies. Several PSMA radiotracers are currently available, such as fluorine-18 (18F) and 68Ga-labeled compounds, with more under investigation, thereby increasing the global availability of PSMA PET imaging[3].
In the treatment of prostate cancer (PCa), the first therapeutic radioactive drug, [177Lu]Lu-PSMA-617 (Pluvicto™), combines a PSMA-specific peptide analog with a therapeutic radioactive nuclide for radioligand therapy. This therapy selectively delivers ionizing radiation to tumor cells, causing their death while sparing surrounding healthy tissues. The efficacy of [177Lu]Lu-PSMA-617 has been confirmed in numerous clinical trials[4]. Approved by the FDA in March 2022 for the treatment of prostate cancer patients, [177Lu]Lu-PSMA-617 (Pluvicto™) has demonstrated high efficacy and low side effects. This success has enabled researchers to explore the tremendous potential of radionuclide therapy further, aiming to develop even more effective drugs.
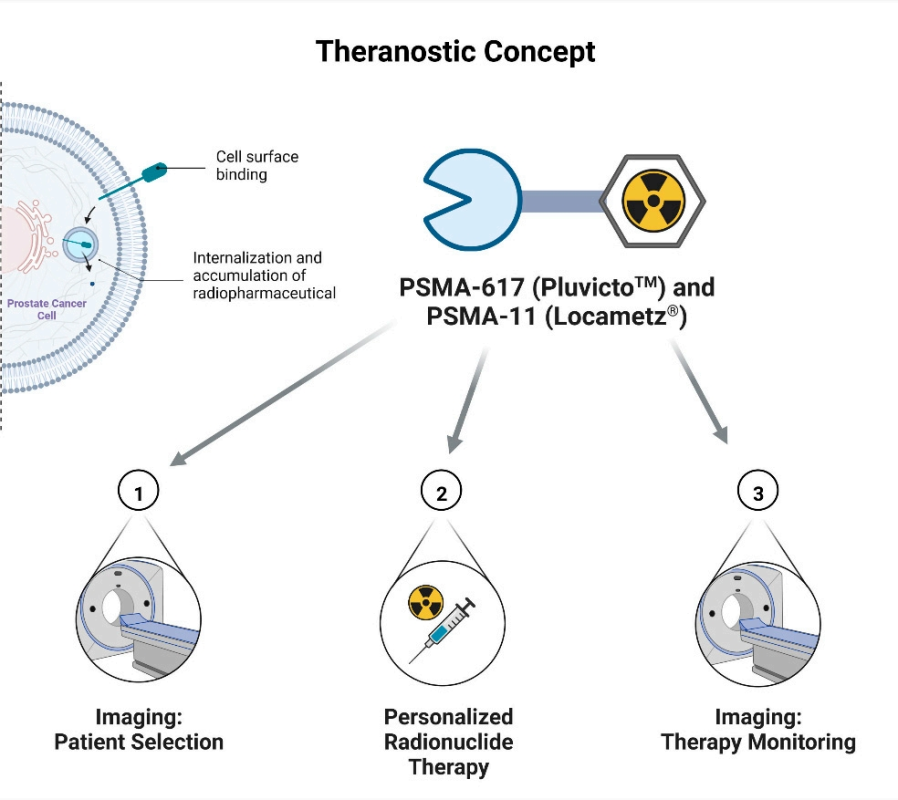
Figure 2.Theranostic concept of Pluvicto.
(Cited fromThe First FDA-Approved Radiotherapeutical for Treatment of Prostate Cancer. Pharmaceuticals (Basel), 2022.)
3. Radiopharmaceuticals Targeting PSMA
According to recent statistics, there are currently 162 radiopharmaceuticals targeting PSMA worldwide. Among these, 74 are in clinical stages, encompassing both diagnostic and therapeutic radiopharmaceuticals. Notably, five of these drugs have already been approved for market use.
3.1 Approved Drugs
Among the approved drugs, 4 are diagnostic radiopharmaceuticals and 1 is a therapeutic radiopharmaceutical, accounting for 80% and 20% of the total, respectively.
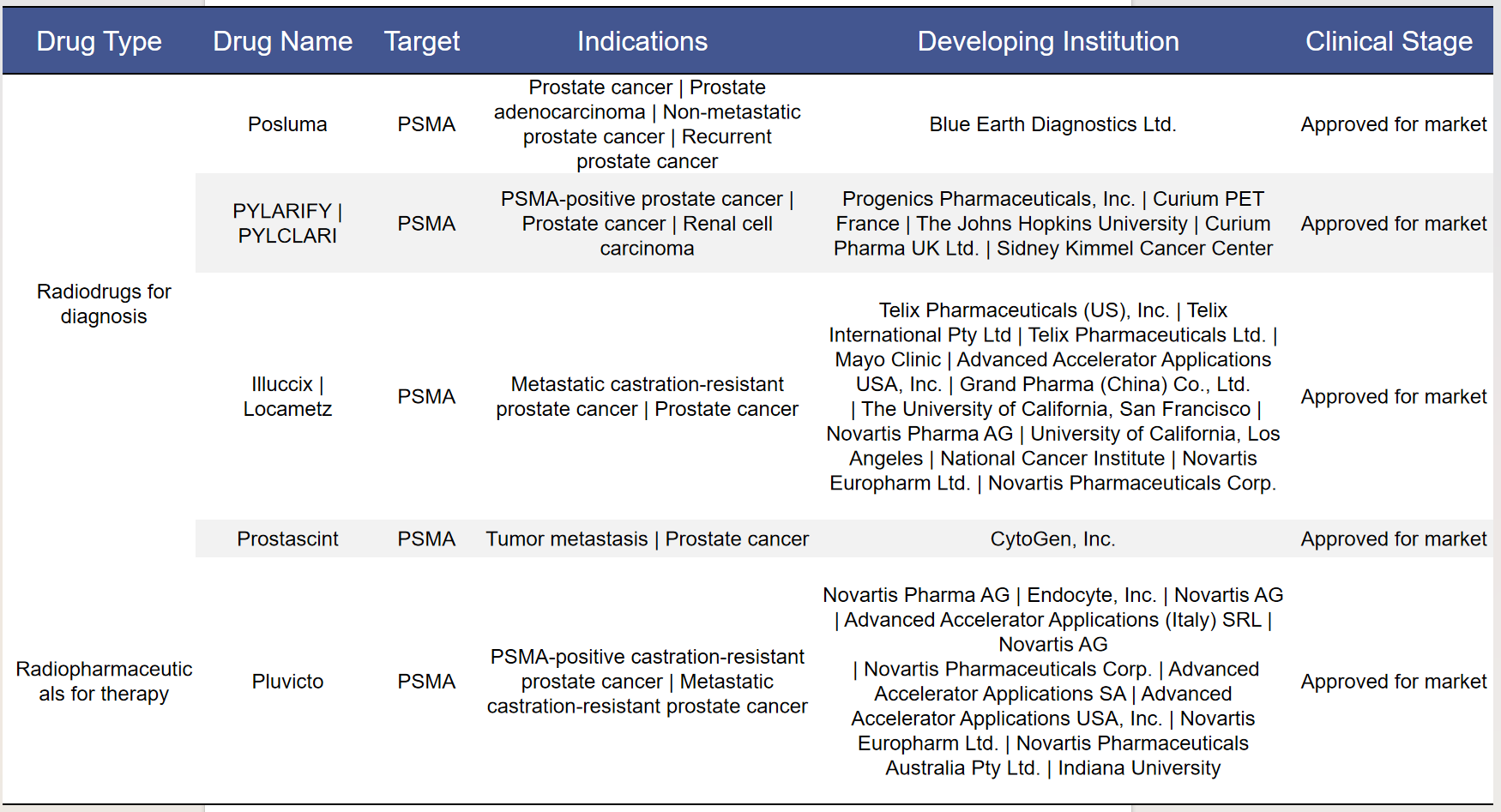
Pluvicto ([177Lu]Lu-PSMA-617) is a highly specific drug that targets prostate-specific membrane antigen (PSMA) overexpressed in prostate tumor tissue. Because PSMA is expressed at low levels in non-prostate tissues, the background accumulation in healthy tissues is minimal, thereby reducing severe side effects and making this therapy safe with low toxicity. Compared to chemotherapy, Pluvicto is highly specific to prostate cancer, thereby limiting damage to surrounding tissues.
3.2 Radiopharmaceuticals in Clinical Stages
- Diagnostic Radiopharmaceuticals
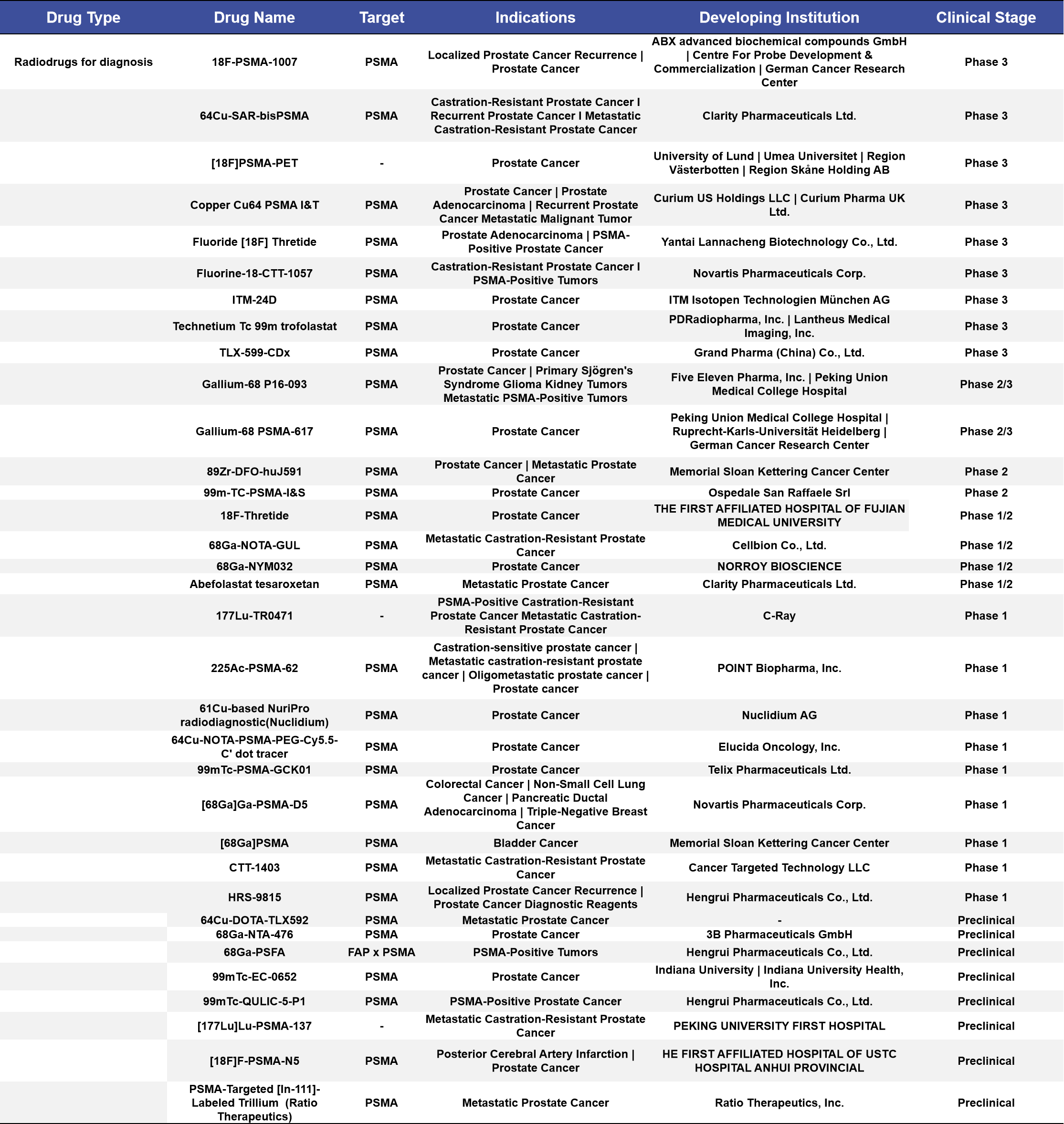
More than half of the diagnostic radiopharmaceuticals in clinical stages are concentrated in Phase 1 and Phase 3 clinical trials, accounting for 52%. Among the 18 targeted indications, the top three are prostate cancer, metastatic castration-resistant prostate cancer (mCRPC), and metastatic prostate cancer, with 13, 4, and 2 drugs targeting these conditions, respectively, accounting for 38%, 11%, and 5.9% of the total. (See Appendix 1 for details)
- Therapeutic Radiopharmaceuticals
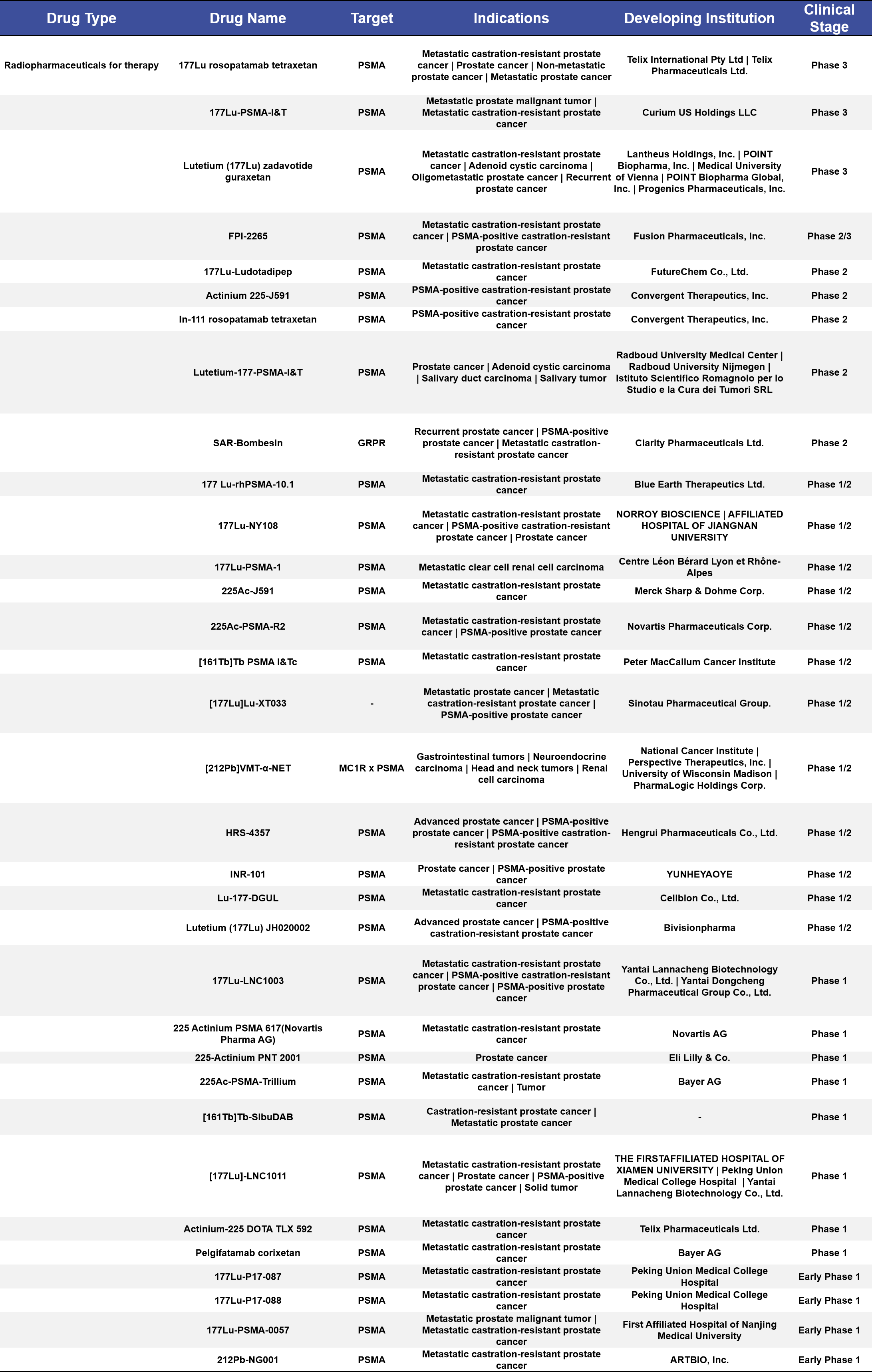
More than half of the therapeutic radiopharmaceuticals in clinical stages are concentrated in Phase 1/2 and Phase 1 trials, accounting for 60%. Among the 22 targeted indications, the top three are metastatic castration-resistant prostate cancer (mCRPC), PSMA-positive castration-resistant prostate cancer, and prostate cancer, with 11, 2, and 1 drugs targeting these conditions, respectively, accounting for 33%, 6.1%, and 3.0% of the total. Most of these drugs use [177Lu] as the radioactive label in the compounds. (See Appendix 2 for details)
4. PSMA-Related Products Supporting Drug Development
Dima Biotechnology is a biotechnology company focused on preclinical research products and services for druggable targets. We currently offer a range of off-the-shelf products targeting the PSMA (Prostate-Specific Membrane Antigen) target, including recombinant proteins, reference antibodies, biotin/PE-fluorescently labeled antibodies, and stable transfected cell lines. Additionally, we provide a comprehensive set of services, including custom protein/antibody services, antibody humanization, antibody affinity maturation, and stable transfected cell line generation.
Furthermore, we have established a B-cell seed bank for the PSMA target, enabling us to screen lead antibody molecules in just 28 days to meet customer needs. For more information, feel free to contact us.
4.1 Proteins&Antibodies&Stable cell lines
| Product Type | Cat No. | Product Name |
| Recombinant protein | PME100390 | Human PSMA Protein, hFc Tag |
| PME100545 | Human PSMA Protein, His Tag | |
| PME100546 | Human PSMA Protein, mFc Tag | |
| PME-M100103 | Mouse PSMA Protein, His Tag | |
| PME-C100035 | Cynomolgus PSMA Protein, His Tag | |
| Reference antibody | BME100128 | Anti-PSMA(rosopatamab biosimilar) mAb |
| Biotin-labeled antibody | BME100128B | Biotinylated Anti-PSMA(rosopatamab biosimilar) mAb |
| PE-conjugated antibody | BME100128P | PE-conjugated Anti-PSMA(rosopatamab biosimilar) mAb |
| Stable cell lines | CEL100019 | Hu_PSMA K562 Cell Line |
4.2 DIMA’s Research Progress on PSMA Lead Molecules
- Appendix
Appendix 1. Diagnostic Radiopharmaceuticals
Appendix 2. Therapeutic Radiopharmaceuticals
References:
1. Chen, J., et al., Current role of prostate-specific membrane antigen-based imaging and radioligand therapy in castration-resistant prostate cancer. Front Cell Dev Biol, 2022. 10: p. 958180.
2. Jones, W., et al., PSMA Theranostics: Review of the Current Status of PSMA-Targeted Imaging and Radioligand Therapy. Cancers (Basel), 2020. 12(6).
3. Farolfi, A., et al., Current and Emerging Clinical Applications of PSMA PET Diagnostic Imaging for Prostate Cancer. J Nucl Med, 2021. 62(5): p. 596-604.
4. Hennrich, U. and M. Eder, [(177)Lu]Lu-PSMA-617 (Pluvicto(TM)): The First FDA-Approved Radiotherapeutical for Treatment of Prostate Cancer. Pharmaceuticals (Basel), 2022. 15(10).