The B cell maturation antigen (BCMA), a target that gained prominence due to its association with multiple myeloma, has recently shown significant progress in the treatment of autoimmune diseases. On May 10, 2024, a research team led by Wang Wei from the Department of Neurology at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, published a study in Science Immunology titled “Single-cell analysis of anti-BCMA CAR T cell therapy in patients with central nervous system autoimmunity.” The study reveals the mechanisms by which anti-BCMA CAR-T cell therapy affects relapsed/refractory neuromyelitis optica spectrum disorder (NMOSD). Most B cells in cerebrospinal fluid (CSF) are central nervous system (CNS) resident B cells, making them potential targets for CNS autoimmune diseases. Today, we will review the clinical research progress of BCMA as a potential treatment target for autoimmune diseases. Before diving into the clinical research advancements, let’s briefly revisit the biological background of the BCMA target.
1. Structure and Distribution of BCMA
Human BCMA, also known as TNFRSF17, CD269, or TNFRSF13A, is encoded by the TNFRSF17 gene located on the short arm of chromosome 16 (16p13.13). This gene is 2.92 kb in length and comprises three exons and two introns. The BCMA protein is a type III transmembrane glycoprotein consisting of 184 amino acids, with a molecular weight of 20.2 kDa.
The BCMA protein structure includes an extracellular domain (ECD) at the N-terminus, a transmembrane domain (TM), and an intracellular domain (ICD). The extracellular N-terminus lacks a signal sequence and contains a conserved motif with six cysteine residues. The ECD is composed of 54 amino acids, the TM domain contains 23 amino acid residues, and the ICD consists of 107 amino acids. In addition to the membrane-bound form (mBCMA), there is a soluble form of BCMA (sBCMA) that results from γ-secretase cleavage. Notably, sBCMA retains the extracellular domain and part of the transmembrane region [3].
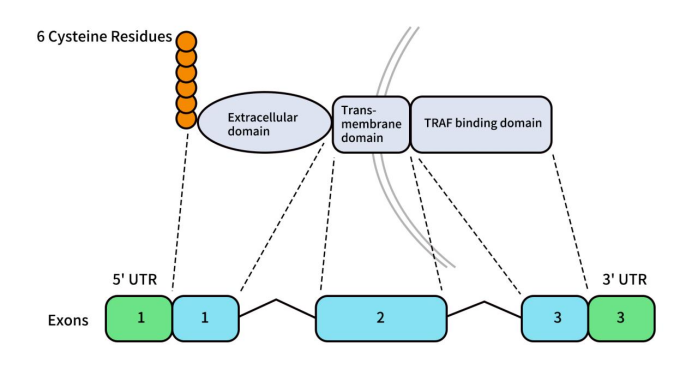
Figure 1. The structures of BCMA gene and protein [1]
In normal tissues, BCMA expression is limited to a small subset of immune cells, including plasma cells, memory B cells within the bone marrow and peripheral organs (such as the gastrointestinal tract, trachea, spleen, and lymph nodes), as well as activated plasma cell-like dendritic cells. BCMA is primarily expressed by mature B lymphocytes, with minimal expression in hematopoietic stem cells or non-hematopoietic tissues. It plays a crucial role in the survival of long-lived bone marrow plasma cells (PC) [4].
2. Research Progress on Targeting BCMA in Autoimmune Diseases
Autoimmune diseases are a heterogeneous group of disorders characterized by the breakdown of immune tolerance, leading to the sensitization of T and B cells against self-antigens and the production of autoantibodies that trigger organ damage. Soluble BCMA (sBCMA) is a potential biomarker for B cell involvement in human autoimmune diseases. Common autoimmune diseases include multiple sclerosis, N-methyl-D-aspartate receptor (NMDAR) encephalitis, myasthenia gravis, systemic lupus erythematosus (SLE), rheumatoid arthritis, myelin oligodendrocyte glycoprotein spectrum disorder (MOGSD), and neuromyelitis optica spectrum disorder (NMOSD).
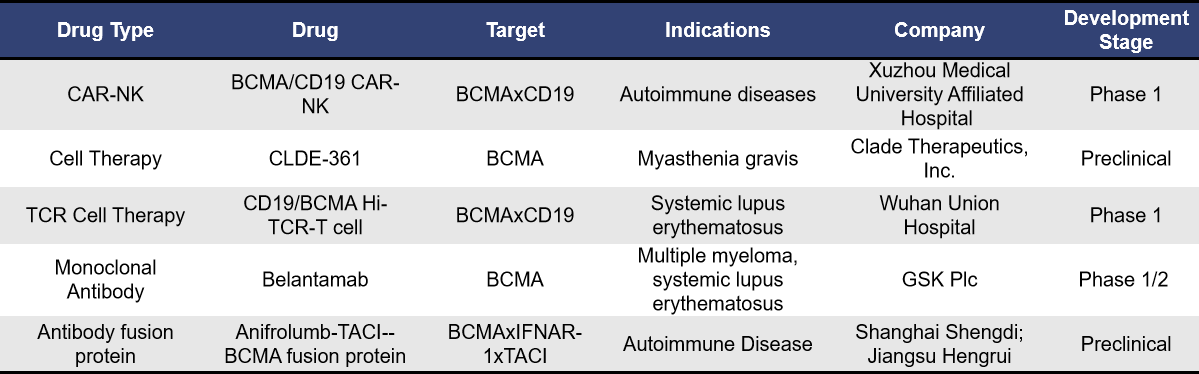
Currently, there are approximately 20 therapies globally targeting BCMA for autoimmune diseases, with most in early clinical stages and primarily focusing on systemic lupus erythematosus. Among these, the most advanced clinical research is Descant-08, an autologous CAR-T therapy developed by Cartesian Therapeutics. The types of therapies being explored include CAR-T, universal CAR-T, autologous CAR-T, CAR-NK, and monoclonal antibodies.
2.1 Targeting BCMA CAR-T Therapies
Currently, there are 7 BCMA-targeting CAR-T therapies under development for autoimmune diseases. Among these, 3 are in Phase 1 clinical trials, 1 is in early Phase 1 clinical trials, and 2 are in preclinical stages.
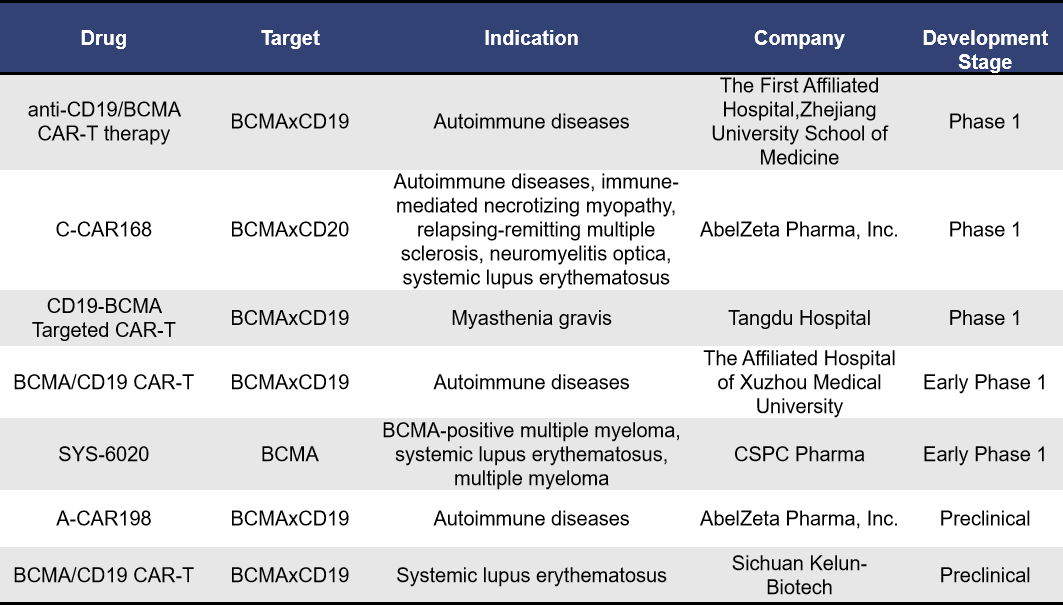
- anti-CD19/BCMA CAR-T therapy (Zhejiang University)
The anti-CD19/BCMA CAR-T therapy conducted by The First Affiliated Hospital of Zhejiang University School of Medicine is currently underway in China. The study is titled “Clinical Research on the Safety and Efficacy of CD19/BCMA CAR-T Cell Therapy for Autoantibody-Mediated Autoimmune Diseases and HLA Antibody Positive Status Before Allogeneic Hematopoietic Stem Cell Transplantation.” The trial ID is ChiCTR2100049920. It began on March 1, 2020, with the first announcement on August 10, 2021. No further details have been disclosed officially.
- C-CAR168&AbelZeta
C-CAR168 is a CD20/BCMA bispecific CAR T-cell injection developed by AbelZeta. It is designed for the treatment of autoimmune diseases. The molecular structure of C-CAR168 has been carefully optimized, and the efficacy and safety of its single-chain variable fragment (scFv) have undergone rigorous clinical testing, demonstrating high efficacy in preclinical studies. Currently, C-CAR168 is being investigated in an exploratory clinical trial (NCT06249438) for patients with autoimmune diseases that are unresponsive to standard therapies (CAR-AID). This study includes conditions such as systemic lupus erythematosus (SLE), immune-mediated necrotizing myopathy (IMNM), neuromyelitis optica spectrum disorder (NMOSD), and multiple sclerosis (MS). The trial started on March 20, 2024.
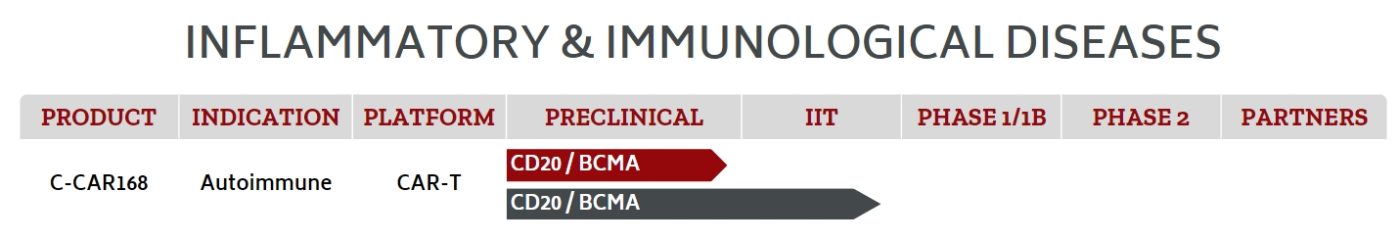
Figure 2. The progress of C-CAR168 (data derived from AbelZeta website)
- SYS6020 & CSPC Pharma
SYS6020 is the world’s first mRNA-LNP-based CAR-T cell therapy candidate approved for clinical trials, and it is CSPC Pharma’s first cell therapy product under development. Compared to traditional CAR-T therapies, SYS6020 offers several advantages, including high cell viability, a high CAR positivity rate, and reduced risks of gene integration-induced tumorigenesis and cytokine storm. Preclinical studies have demonstrated that SYS6020 can significantly target and kill BCMA-positive multiple myeloma cells, showing promising safety and efficacy. In August 2024, SYS6020 was approved for clinical use in China, with plans to treat refractory active systemic lupus erythematosus.
2.2 Universal BCMA-Targeting CAR-T
Universal CAR-T cell therapy involves isolating T cells from healthy donors, which are then genetically edited or modified without genetic editing and expanded in vitro. The final product can be administered to multiple patients. Currently, for autoimmune diseases, there are mainly 2 universal BCMA-targeting CAR-T therapies, with BCMA-UCART from Shanghai Bangyao Biotechnology being a representative example.
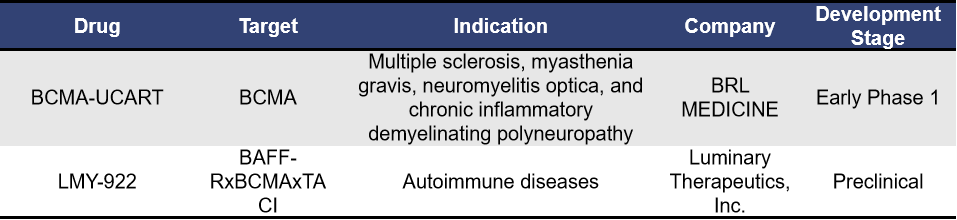
BCMA-UCART is a universal CAR-T product targeting BCMA developed by BRL MEDICINE using its universal cell platform, TyUCell®. This platform involves genetic editing of allogeneic immune cells to eliminate immune rejection, achieving universal applicability while ensuring the safety and efficacy of the cell therapy products. BCMA-UCART is currently in the IIT research evaluation stage, focusing on autoimmune diseases such as multiple sclerosis and myasthenia gravis.
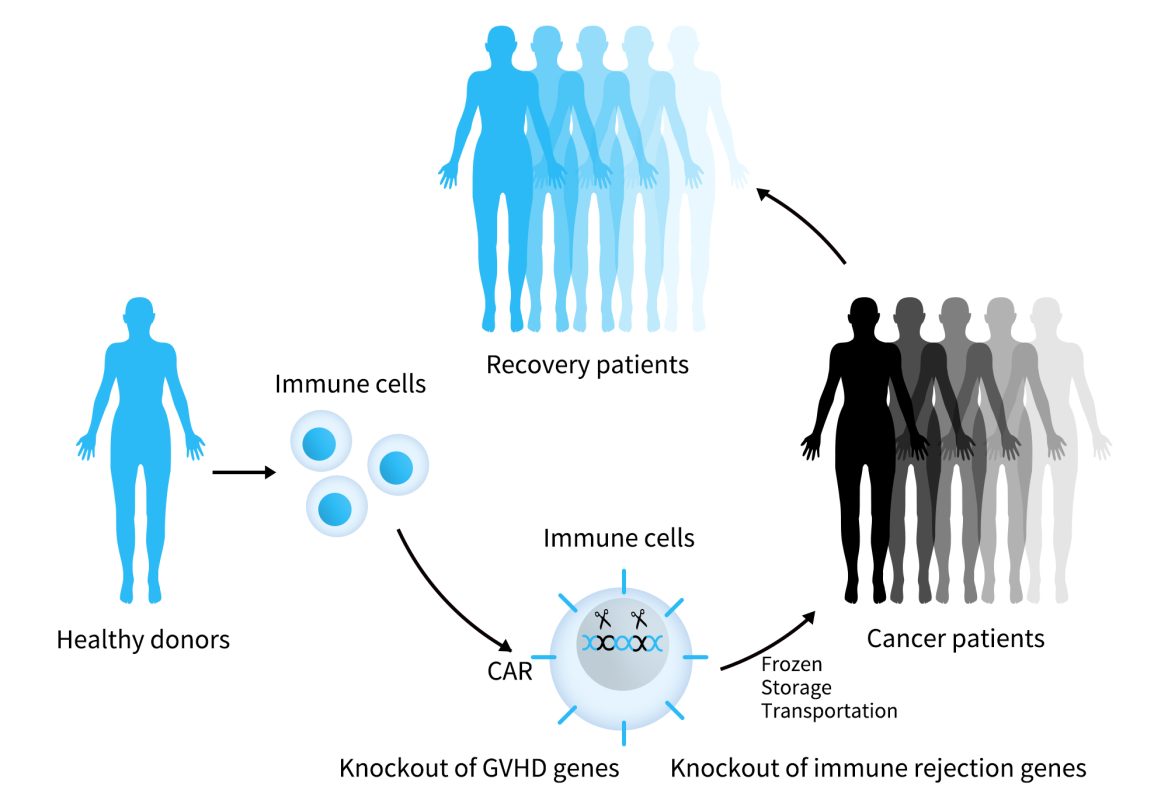
Figure 3. the process of UCART (data derived from BRL MEDICINE website)
2.3 BCMA-Targeted Autologous CAR-T
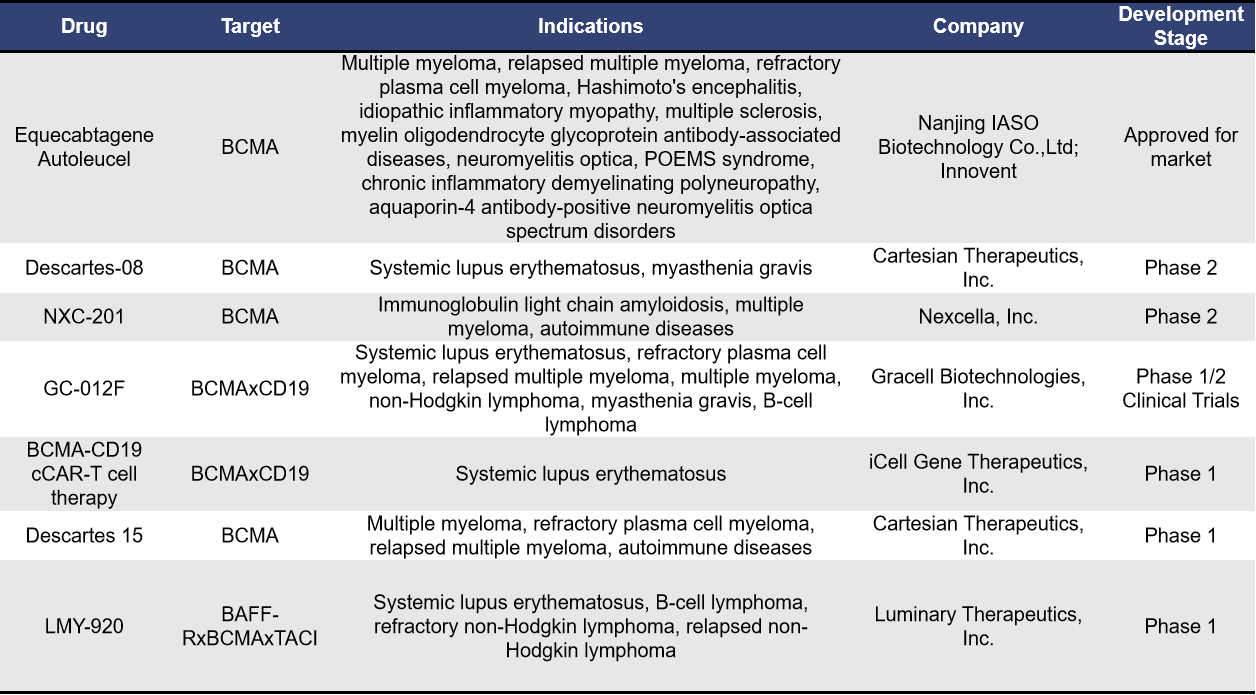
Autologous CAR-T, as the name suggests, involves using T cells derived from the patient’s own body. Currently, there are 7 BCMA-targeted autologous CAR-T therapies for autoimmune diseases. Among them, Descartes-08 and NXC-201 are progressing rapidly in clinical research. Although Equecabtagene Autoleucel has already been approved, its clinical research for autoimmune diseases is still in the Phase 1 stage (NCT04561557).
- Descartes-08&Cartesian Therapeutics
Descartes-08, developed by Cartesian Therapeutics, is an autologous mRNA CAR-T therapy targeting BCMA. Unlike traditional DNA CAR-T cell therapies, mRNA CAR-T administration does not require chemotherapy preconditioning, effectively avoiding risks associated with genomic integration and cancer metastasis. Cartesian is currently conducting Phase 2 clinical trials for Descartes-08 in patients with generalized myasthenia gravis (NCT04146051) and systemic lupus erythematosus (SLE) (NCT06038474). Previously, this drug received FDA Orphan Drug Designation and Regenerative Medicine Advanced Therapy (RMAT) designation for the treatment of myasthenia gravis.
On July 2, 2024, Cartesian Therapeutics announced that the Phase IIb trial for Descartes-08 in myasthenia gravis achieved positive top-line results (NCT04146051), meeting the statistically significant primary endpoint. On the same day, Cartesian also reported that the Phase 2 trial for Descartes-08 in systemic lupus erythematosus (NCT06038474) had dosed its first patient. This open-label Phase 2 trial aims to enroll approximately 30 adult patients to evaluate the safety and tolerability of Descartes-08 administered in an outpatient setting without chemotherapy preconditioning in moderate to severe SLE patients who are refractory to immunosuppressive therapy.
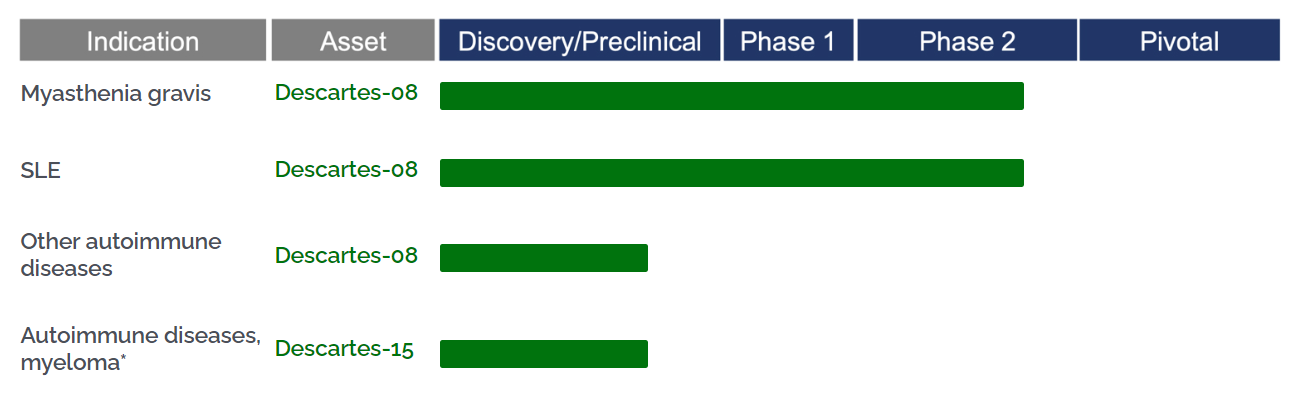
Figure 4. The process of Descartes-08 and Descartes-15 (data derived from Cartesian Therapeutics website)
In addition to Descartes-08, Cartesian Therapeutics is also developing Descartes-15, an autologous BCMA-targeted CAR-T cell therapy. Plans are underway to initiate the first-in-human Phase 1 dose escalation trial to assess the safety and tolerability of Descartes-15 in patients with multiple myeloma. For autoimmune disease indications, Descartes-15 is still in the preclinical stage.
- XC-201&Nexcella
NXC-201 (formerly known as HBI0101) is a stereotactically optimized BCMA-targeted CAR-T cell therapy, also an autologous CAR-T therapy using the patient’s own cells, designed to seek out and destroy malfunctioning plasma cells responsible for these diseases. NXC-201 has the potential to become the only “single-day CRS” CAR-T therapy for AL amyloidosis and other autoimmune diseases. It has received Orphan Drug Designation (ODD) from the FDA for AL amyloidosis and multiple myeloma, and from the EMA for AL amyloidosis. A Phase 1b dose-expansion clinical trial of NXC-201 is expected to take place in the United States, targeting relapsed/refractory AL amyloidosis patients with good cardiac function who have not previously received BCMA-targeted therapy (NCT06097832). This study began on June 5, 2024, and is currently in the recruitment phase.
- GC012F&Gracell Biotechnologies
GC012F is a BCMA + CD19 dual-target autologous CAR-T cell therapy developed using Gracell Biotechnologies Ltd.’s FasTCAR technology platform. This platform aims to significantly improve patient outcomes by enhancing efficacy, reducing production costs, and increasing the accessibility of CAR-T therapies. The FasTCAR technology platform streamlines the three main production steps of autologous CAR-T cells—activation, transduction, and expansion—into a single step of “synchronous activation-transduction.” By minimizing the duration of ex vivo culture, FasT CAR-T cells have a younger phenotype, enhanced expansion capacity, and greater tumor cell clearance activity. This approach reduces the required dosage and eliminates the need for ex vivo expansion, allowing for optimal in vivo expansion within the patient.
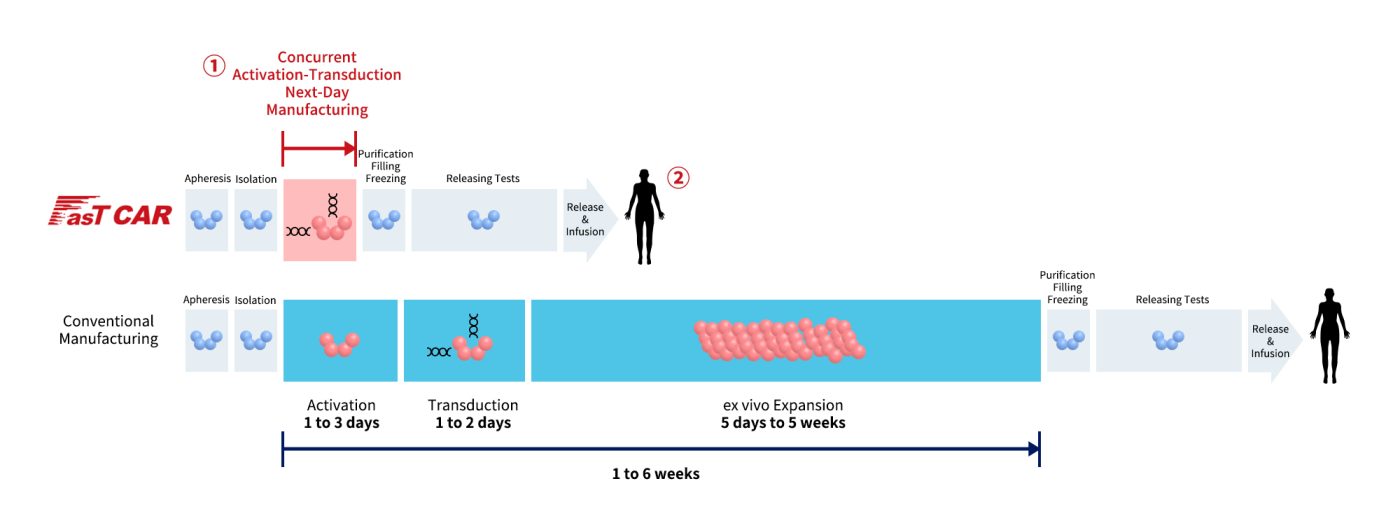
Figure 5. The comparison of FasT CAR-T and conventional Manufacturing (data derived from Gracell Biotechnologies website)
Currently, Gracell Biotechnologies Ltd. is conducting multiple clinical trials for GC012F, with the most advanced research focusing on multiple myeloma. According to ClinicalTrials.gov, GC012F is undergoing clinical trials for refractory myasthenia gravis and refractory systemic lupus erythematosus (SLE), with trial identifiers NCT06419166 and NCT06530849, respectively. NCT06419166 is an exploratory clinical trial assessing the efficacy of GC012F in treating refractory generalized myasthenia gravis. NCT06530849 is a Phase 1/2 clinical trial designed to evaluate the safety and efficacy of GC012F in patients with refractory SLE.
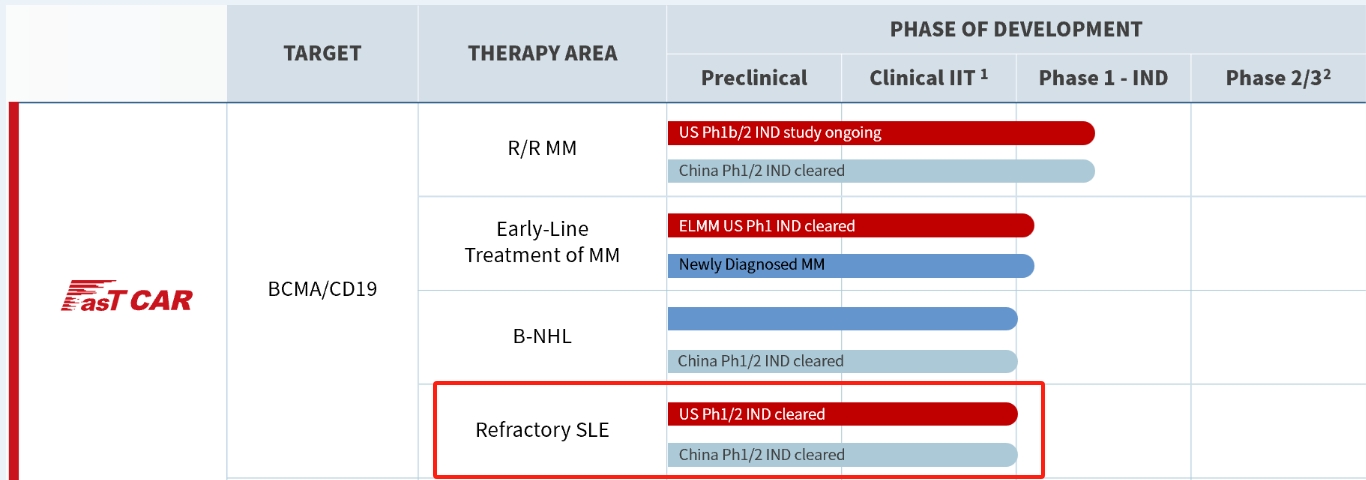
Figure 5. The process of GC012F (data derived from Gracell Biotechnologies website)
- BCMA-CD19 cCAR-T cell therapy&iCell Gene Therapeutics
BCMA-CD19 cCAR-T cell therapy is a BCMA-CD19 composite CAR-T therapy (cCAR-T) developed by iCell Gene Therapeutics. This therapy aims to simultaneously target the plasma cell antigen BCMA and the memory B cell antigen CD19, thereby achieving comprehensive depletion of B cells in patients. The goal is to enable immune reset, symptom relief, and disease remission without further medication.
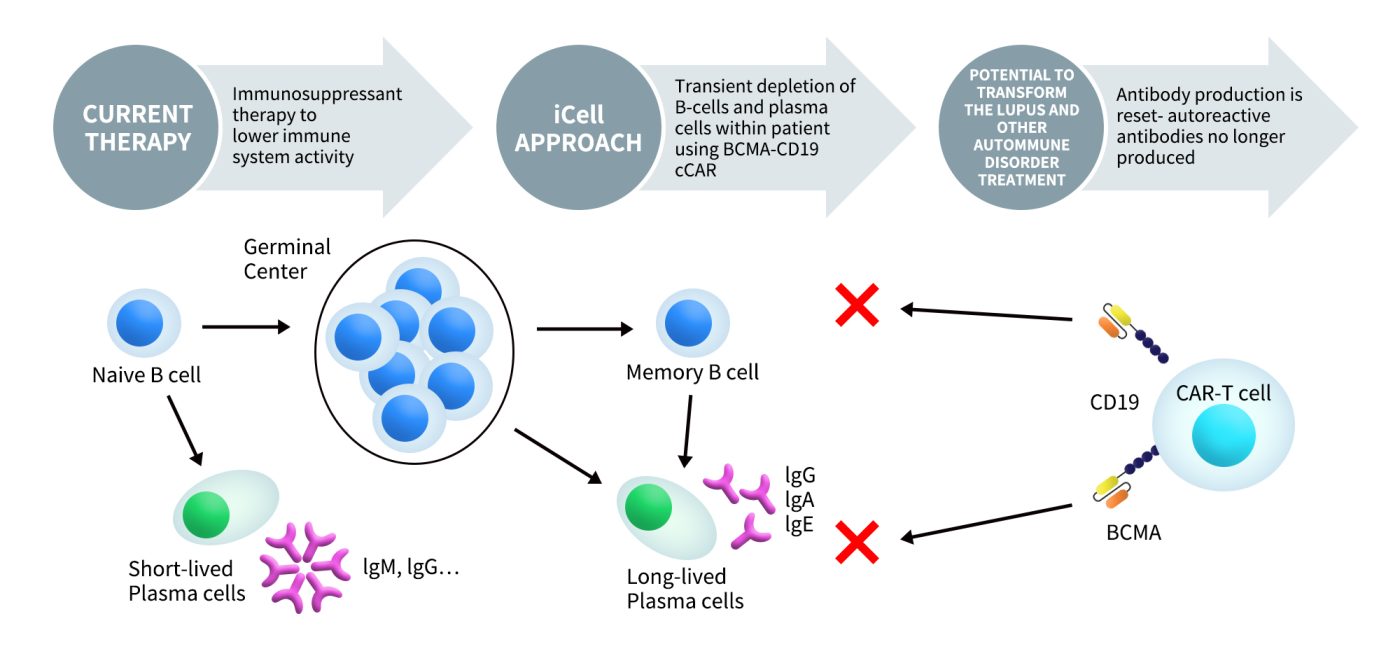
Figure 6. The mechanism of iCell Gene’s BCMA-CD19 cCAR-T (data from iCell Gene website)
iCell Gene Therapeutics is currently conducting an open-label, multi-center Phase I clinical trial for BCMA-CD19 cCAR-T cell therapy, aimed at assessing the safety and tolerability of this treatment in patients with refractory and/or relapsing systemic lupus erythematosus (SLE) (NCT05474885). The trial started on April 1, 2022, and is still recruiting participants. On May 27, 2024, iCell Gene Therapeutics reported positive results from the clinical trial of BCMA-CD19 cCAR-T cell immunotherapy. The results indicated that all SLE/lupus nephritis (LN) patients who received the full initial dose of cCAR-T treatment had negative autoantibody levels three months post-treatment, and by the 46-month follow-up, they all achieved symptom-free and medication-free remission (MFR).
2.4 Other therapies targeting BCMA
In addition to universal CAR-T and autologous CAR-T therapies, other targeted BCMA therapies in development for autoimmune diseases include CAR-NK cells, monoclonal antibodies, and antibody fusion proteins.
- CLDE-361&Clade Therapeutics
CLDE-361, developed by Clade Therapeutics, is an αβ iT cell project targeting BCMA in severe myasthenia gravis muscle disease, currently in the preclinical stage with no further information disclosed. Clade provides a technology to precisely control iPSC differentiation to produce adaptive αβ CD4+ and CD8+ T cells for treating cancer and autoimmune diseases. In April 2024, Century Therapeutics announced the acquisition of Clade Therapeutics Inc. to enhance its product line and platform.
3. DIMA Biotechnology: Advancing BCMA Biotherapy Development
DIMA Biotechnology is a biotechnology company specializing in preclinical development products and services for druggable targets. DIMA currently offers a comprehensive range of BCMA-targeted products and services, including active proteins, reference antibodies, and flow cytometry validation monoclonal antibodies. Services provided include customized protein and antibody development, antibody humanization, and affinity maturation. To accelerate the development of BCMA-based biologics, DIMA has also established a BCMA-targeted single B-cell seed bank, with lead antibody molecules available in as little as 28 days. We have identified several BCMA lead antibody molecules and are conducting CAR-T or ADC molecular construction and functional validation for some of these lead antibodies. For detailed data, please feel free to inquire.
- Recombinant Protein&Antibody&Stable Cell Line&CDX Slide
- Progress on BCMA Lead mAb Molecules

Reference:
[1]Yu, B., Jiang, T. & Liu, D. BCMA-targeted immunotherapy for multiple myeloma. J Hematol Oncol, 2020, 13, 125.
[2]Bera, T.K. Anti-BCMA Immunotoxins: Design, Production, and Preclinical Evaluation. Biomolecules 2020, 10, 1387.
[3]Laurent, S. A., Hoffmann, F. S., Kuhn, P. H., et al. γ-Secretase directly sheds the survival receptor BCMA from plasma cells. Nature communications, 2015, 6, 7333.
[4]Shah, N., Chari, A., Scott, E. et al. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia, 2020, 34, 985–1005.
