Seizure related 6 homolog (SZE6) is a transmembrane protein discovered on the surface of selected neuronal lineage cells. Its expression is limited in normal tissues but highly expressed in small cell lung cancer (SCLC), making it an ideal target for ADC (Antibody-Drug Conjugate) therapy. AbbVie presented early data on their SEZ6 ADC drug, ABBV-706, in the dose escalation part of its first-in-human trial at the 2024 ASCO meeting. The data revealed an overall confirmed objective response rate (ORR) of 43.8% in 48 evaluable patients. In the subset with small cell lung cancer, the confirmed ORR was 60.9%. This isn’t AbbVie’s first foray into SEZ6 ADCs; earlier at the 2023 ASCO meeting, AbbVie showcased Phase I data for ABBV-011, which reported an ORR of 25%, median duration of response of 4.2 months, and median progression-free survival (mPFS) of 3.5 months. However, in August of the same year, AbbVie removed ABBV-011 from its pipeline webpage, signaling the termination of its development. Therefore, ABBV-706 can be considered AbbVie’s second-generation SEZ6-ADC. What is it about SEZ6 that continues to intrigue AbbVie, prompting them to invest in its development time and again?
1. SEZ6 and the SEZ6 Family
SEZ6, encoded by the SEZ6 gene located on chromosome 17q11.2, belongs to the SEZ6 family. The other two members of the SEZ6 family are seizure-related 6-like protein (SEZ6L) and seizure-related 6-like protein 2 (SEZ6L2). SEZ6L and SEZ6L2 share over 40% identity and 60% similarity with SEZ6. SEZ6 undergoes mRNA alternative splicing to generate different SEZ6 isoforms: two of them (Sez6 type I and type II) are cell surface proteins with a single transmembrane domain (type I transmembrane), while Sez6 type III is a secreted isoform (sSez6). Structural domains of SEZ6 family members include 5 sushi domains and 2-3 CUB domains. Sushi domains, also known as complement control protein (CCP) domains or short consensus repeat (SCR) domains, consist of 60-70 amino acids with four conserved cysteine residues forming disulfide bonds. These domains are involved in various recognition processes, including binding of several complement factors to C3b and C4b fragments. CUB domains (complement C1r/C1s, Uegf, Bmp1) are structural motifs of approximately 110 residues found predominantly in extracellular and membrane-associated proteins, many of which are regulated during development. Additionally, the C-terminal domain of SEZ6 is expected to contain an NPxY binding motif, typically involved in protein internalization and reported to be crucial for SEZ6L2 internalization [1][2].
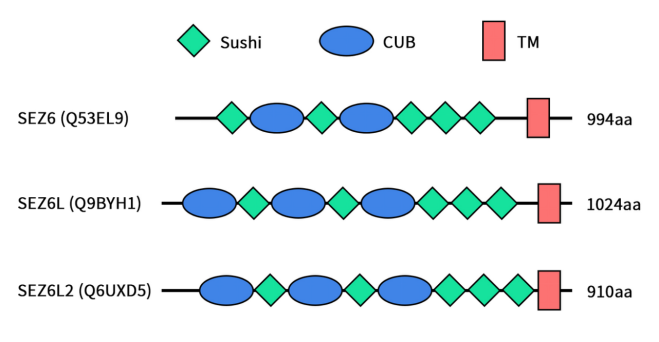
Figure 1. The structures of SEZ6 family
2. SEZ6 Distribution and Functions
SEZ6, SEZ6L, and SEZ6L2 exhibit distinct spatial and temporal expression patterns. Among them, SEZ6 is primarily expressed in neurons, with highest expression observed in the developing and postnatal neocortex. In the adult mouse brain, SEZ6 expression decreases but remains detectable in the mid-cortical layers, olfactory tubercle, cingulate cortex, and hippocampus (particularly in CA1) [3].
Despite SEZ6 gene knockout mice being available for over a decade, the molecular functions of SEZ6 remain largely unknown. Due to the high expression of SEZ6 mRNA in the developing forebrain, SEZ6 is believed to play critical roles in the development and function of the cerebral cortex, hippocampus, and thalamus. Behaviorally, SEZ6 knockout mice exhibit impaired motor coordination, deficits in long-term memory, and reduced anxiety levels [4]. Furthermore, given the structural richness of SEZ6 in sushi and CUB domains—both involved in protein-protein interactions and found in numerous neurotransmitter receptor binding proteins—SEZ6 and its related family members may participate in cell adhesion or the regulation of ionotropic neurotransmitter receptors [5]. These domains, present not only in neurotransmitter receptor binding proteins but also in proteins of the complement pathway, suggest that SEZ6 and its family members may possess activities related to complement cascade regulation.
Research by Wen Q Qiu and colleagues indicates that members of the Sez6 family inhibit C3b/iC3b opsonization via both classical and alternative pathways, albeit to varying degrees. In the classical pathway, Sez6 acts as a potent inhibitor, Sez6L2 as a moderate inhibitor, and Sez6L as a weak inhibitor. In the alternative pathway, the complement inhibitory activities of Sez6, Sez6L, and Sez6L2 are comparable to or greater than those of known complement regulators like MCP (Membrane Cofactor Protein) [6].
3. Clinical Drug Development Progress of SEZ6
SEZ6 is involved in dendrite formation and regulates neuronal signal transmission, closely linking it to the progression of neuroendocrine tumors (NETs). Additionally, its selective high expression on neuroendocrine tumor cells, contrasting with minimal expression in most normal tissues, positions it as a potential drug target for NETs. Currently, there are only two publicly disclosed clinical pipelines worldwide targeting SEZ6: ABBV-011 and ABBV-706, both developed by AbbVie. However, ABBV-011 was discontinued by AbbVie in August 2023.
ABBV-706 is an ADC targeting SEZ6, coupled with a topoisomerase 1 inhibitor (TOP1i) through a Valine-alanine linker, with a drug-to-antibody ratio (DAR) of 6. ABBV-706 specifically targets tumor cells expressing SEZ6, rapidly internalizes, and delivers the Top1i payload to kill tumor cells effectively. It has demonstrated significant anti-proliferative activity across various small cell lung cancer (SCLC) cell lines and has induced sustained tumor regression in SCLC patient-derived xenograft (PDX) models. Currently, ABBV-706 is undergoing Phase I clinical trials that began on October 31, 2022, with a target of recruiting 350 participants. The trial is scheduled to conclude on December 18, 2026, and recruitment efforts are ongoing.
This is a Phase I first-in-human trial aimed at evaluating ABBV-706’s safety, pharmacokinetics, and efficacy as monotherapy or in combination with budigalimab (ABBV-181), carboplatin, or cisplatin in patients with advanced solid tumors. The trial is divided into four parts:
Part 1 (Dose Escalation): ABBV-706 will be intravenously infused as monotherapy in escalating doses until the maximum tolerated dose (MTD) is determined for patients with small cell lung cancer (SCLC), high-grade CNS tumors, and high-grade neuroendocrine carcinoma (NEC). Part 2: Several doses identified in Part 1 will be selected, and SCLC patients will be randomized to receive one of these doses to establish the recommended Phase 2 dose. Part 3a: SCLC or NEC patients will receive ABBV-706 in combination with budigalimab intravenously every 3 weeks. Part 3b: SCLC or NEC patients will receive ABBV-706 in combination with carboplatin or cisplatin intravenously. Part 4a: Participants with central nervous system tumors will receive ABBV-706 intravenously at the dose determined in Part 1. Part 4b: Participants with NEC will receive ABBV-706 intravenously at the dose selected in Part 1. The study is expected to last up to 3 years.
At the 2024 ASCO conference, AbbVie presented early data from the first-in-human study of ABBV-706 in patients with advanced solid tumors. The summary and speech included statistical data up to November 15, 2023, and March 20, 2024, respectively.
As of November 15, 2023, among 33 evaluable patients, the overall confirmed objective response rate (ORR) was 21% (7 partial responses). Specifically, in patients with small cell lung cancer (SCLC), the ORR was 40% (6/15), and in neuroendocrine neoplasms (NEN), it was 6% (1/18). Major adverse events included anemia (51%), fatigue (41%), neutropenia (31%), and leukopenia (31%), with no incidents of pneumonia/interstitial lung disease reported. The maximum tolerated dose was determined to be 3 mg/kg IV every 3 weeks. The unconfirmed overall response rate was 45%, with rates of 73% in SCLC patients and 22% in NEN patients. The clinical benefit rate was 91%. By March 20, 2024, data from 48 evaluable patients showed a confirmed ORR of 44% (21/48). Specifically, in SCLC patients, the ORR was 60.9% (14/23), and in NEN patients, it was 28% (7/25). Major adverse events included neutropenia (42%), anemia (42%), and leukopenia (28%).
4. DIMA Biotechnology Supports SEZ6 Drug Development
DIMA Biotechnology is a biotechnology company dedicated to preclinical development products and services for all druggable targets. DIMA now offers a full range of products and services related to the SEZ6 target. Our products include active proteins, flow cytometry validated monoclonal antibodies, and stable transfected cell lines. Services encompass a variety of species-specific antibody customization, humanization, and affinity maturation services.
To expedite the development of SEZ6 drug development, DIMA has established SEZ6 target single B cell seed library, enabling the generation of lead antibody molecules in as little as 28 days. Currently, we have identified 17 lead SEZ6 molecules, available for functional evaluation and validation on the second day. Additionally, we are conducting ADC internalization and cytotoxicity validation for selected molecules. For specific data inquiries, please feel free to contact us.
- Recombinant Protein&Antibody&Stable Cell Line
| Product Type | Cat. No. | Product Name |
| Recombinant Protein | PME101194 | Human SEZ6 Protein, His Tag |
| PME-M100083 | Mouse SEZ6 Protein, His Tag | |
| FC-validated Antibody | DMC101096 | Anti-SEZ6 antibody(31A10), IgG1 Chimeric mAb |
| Biotin-labeled Antibody | DMC101096B | Biotinylated Anti-SEZ6 antibody(31A10), IgG1 Chimeric mAb |
| Stable Cell Line | CEL100037 | Hu_SEZ6 K562 Cell Line |
- Progress on SEZ6 Lead mAb Molecules

Reference:
[1]Bonifacino JS, Traub LM (2003) Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annual review of biochemistry 72:395-447.
[2]Boonen M, Staudt C, Gilis F, et al. (2016) Cathepsin D and its newly identified transport receptor SEZ6L2 can modulate neurite outgrowth. Journal of cell science 129 (3):557-568.
[3]https://edoc.ub.uni-muenchen.de/25170/7/Pigoni_Martina.pdf.
[4]Gunnersen JM, Kim MH, Fuller SJ, et al. (2007) Sez-6 proteins affect dendritic arborization patterns and excitability of cortical pyramidal neurons. Neuron 56 (4):621-639.
[5]Lin HH, Stacey M, Saxby C, et al. (2001) Molecular analysis of the epidermal growth factor-like short consensus repeat domain-mediated protein-protein interactions: dissection of the CD97-CD55 complex. The Journal of biological chemistry 276 (26):24160-24169.
Qiu WQ, Luo S, Ma SA, et al. The Sez6 Family Inhibits Complement by Facilitating Factor I Cleavage of C3b and Accelerating the Decay of C3 Convertases. Front Immunol. 2021 Apr 15;12:607641.
