On June 25, 2024, at the 2024 American Diabetes Association (ADA) Scientific Sessions, Suzhou Innovent Biologics and Eli Lilly announced the results of a Phase 3 clinical trialfor the GLP-1R x GCGR dual agonist, Mazdutide. This marks the first Phase 3 clinical trial (GLORY-1) in overweight or obese adults in China。The study’s primary results and exploratory endpoints of liver fat content were disclosed, showing that Mazdutide acts as a dual agonist for both GLP-1R and GCGR. It reduces body weight by suppressing appetite and delaying gastric emptying through GLP-1R activation, while enhancing weight loss efficacy by increasing energy expenditure via GCGR activation. Notably, Mazdutide uniquely promotes fatty acid oxidation and lipolysis in the liver through GCGR activation, thereby improving liver fat metabolism. As GCGR plays a crucial role in glucose metabolism, it has become a hot target in the development of diabetes and weight loss drugs.Let’s delve into the role of the Glucagon Receptor (GCGR).
1.Structure and Distribution of GCGR
The glucagon receptor (GCGR) belongs to the class B G protein-coupledreceptor (GPCR) family[1]. Like other GPCR proteins, GCGR has seven transmembrane domains, including three extracellular loops and three intracellular loops, with a molecular weight of 62 kDa. GCGR, along with some other class B GPCRs such as the glucagon-like peptide-1 receptor (GLP-1R) and the gastric inhibitory peptide receptor (GIPR), features a relatively long extracellular loop 1 (ECL1), containing 11-26 residues. In contrast, other class B GPCRs and most class A GPCRs have only 4-6 residues in ECL1. Studies suggest that the ECL1 of GCGR may be involved in binding with its endogenous ligand, glucagon, and in regulating receptor activity[2]. GCGR is predominantly expressed in the liver and kidneys, with smaller amounts found in the intestinal smooth muscle, brain, adipose tissue, adrenal glands, heart, and α- and β-cells of the pancreas[3].
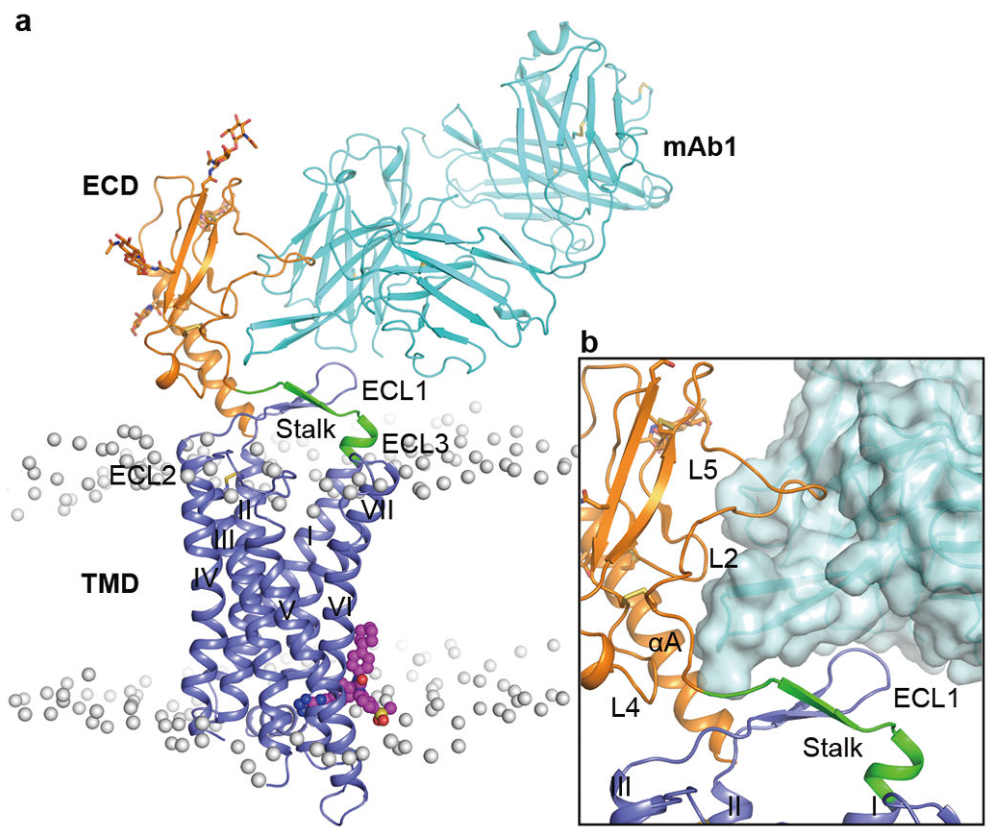
Fig1. Structure of GCGR [2]
2.GCGR-Mediated Signaling Pathways
GCGR plays a critical role in glucose metabolism and glucose homeostasis. When glucagon binds specifically to GCGR, it promotes glycogen breakdown in the liver, increasing blood glucose levels and stimulating insulin release. Studies indicate that under normal physiological glucose concentrations, GCGR can enhance insulin secretion in response to glucagon [4]. As a member of the class B GPCR family, GCGR is involved in various physiological processes and is a valuable drug target for a range of diseases, including diabetes, metabolic syndrome, osteoporosis, migraine,depression, and anxiety[2], with a particularly important role in the treatment of type 2 diabetes. Glucagon binds to GCGR on pancreatic β-cells, and the activated receptor then binds to the G protein Gα, leading to the activation of adenylate cyclase (AC) and the formation of cAMP, which results in increased blood glucose levels[5].
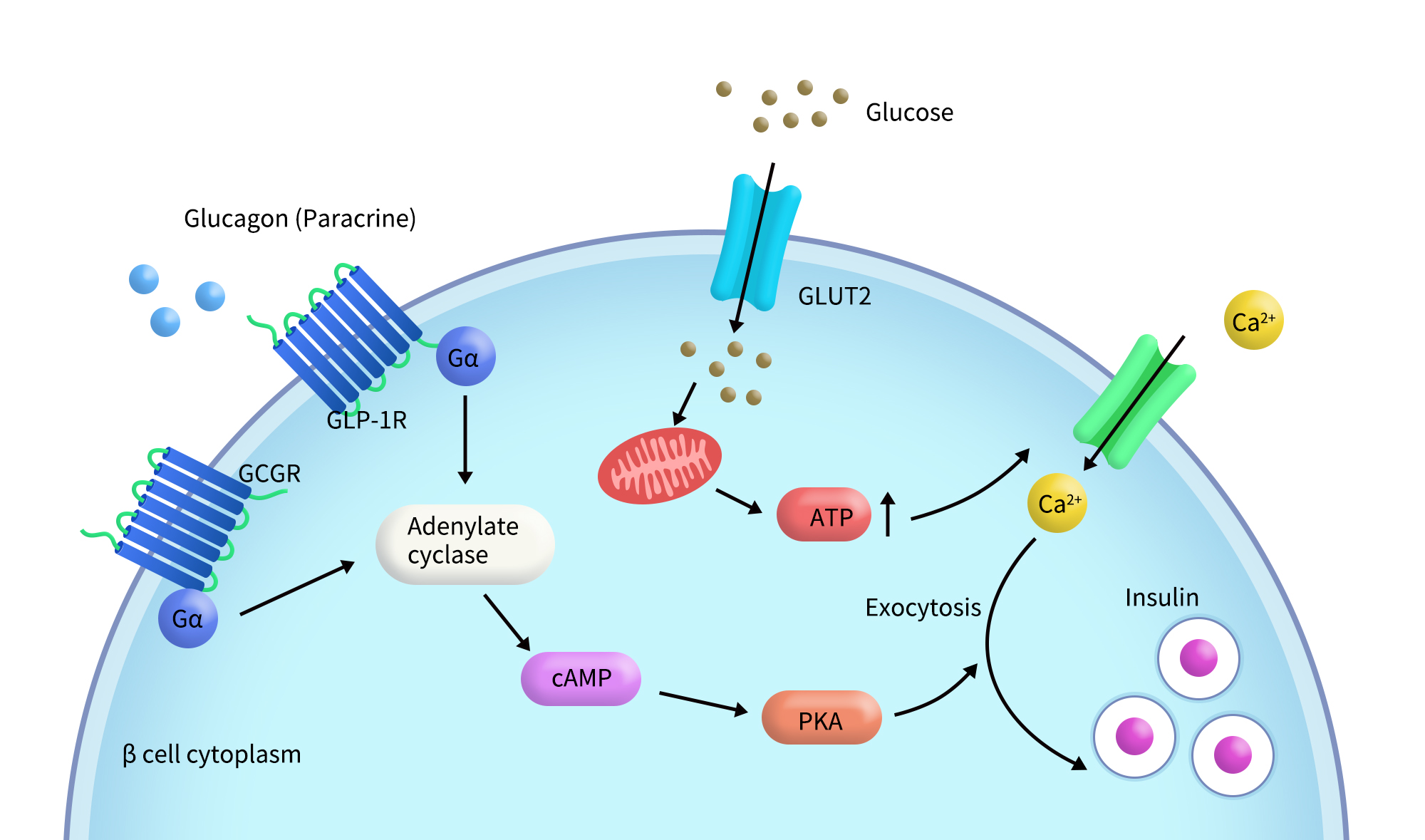
Fig2. GCGR-mediated signaling pathway in pancreatic β cells [5]
3.Clinical Research Progress of GCGR-Targeted Drugs
According to incomplete statistics, there are currently 103 drugs targetingGCGR worldwide, but most are either in preclinical stages or have shown no progressAmong the drugs in clinical stages, there are 8 peptide-based drugs, 2 small molecule drugs, and 7 other types, including fusion proteins, monoclonal antibodies, and hormones. Most of these drugs are intended for the treatment of diabetes and obesity. However, only 5 drugs specifically target GCGR , with just 2 of these having been approved for the market, both of which are hormone-based drugs.
- Synthetic peptides
As of now, there are 8 synthetic peptide drugs targeting GCGR in clinical development. These drugs primarily address obesity and diabetes and include dual agonists for GCGR and GLP-1R, as well as triple agonists for GCGR, GIPR, and GLP-1R. Among them, only the GLP-1R x GCGR dual agonist, Mazdutide, developed by Innovent Biologics/Eli Lilly, has completed clinical trials and is seeking market approval. Two drugs are in Phase 3 clinical trials: Eli Lilly’s GCGR x GIPR x GLP-1R triple agonist, Retatrutide, and Boehringer Ingelheim’s GCGR x GLP-1R dual agonist, Survodutide. Three drugs are in Phase 2 trials: AstraZeneca’s GCGR x GLP-1R dual agonist, Cotadutide; Merck’s GCGR x GLP-1R dual agonist, Efinopegdutide; and a collaboration between Tuweian and Sun Yat-sen University on the GCGR x GLP-1R dual agonist, TB-001. Two drugs are in Phase 1 trials: PegBio’s GCGR x GLP-1R dual agonist, PB-718, and United Bio-Technology’s GCGR x GIPR x GLP-1R triple agonist,UBT-251.
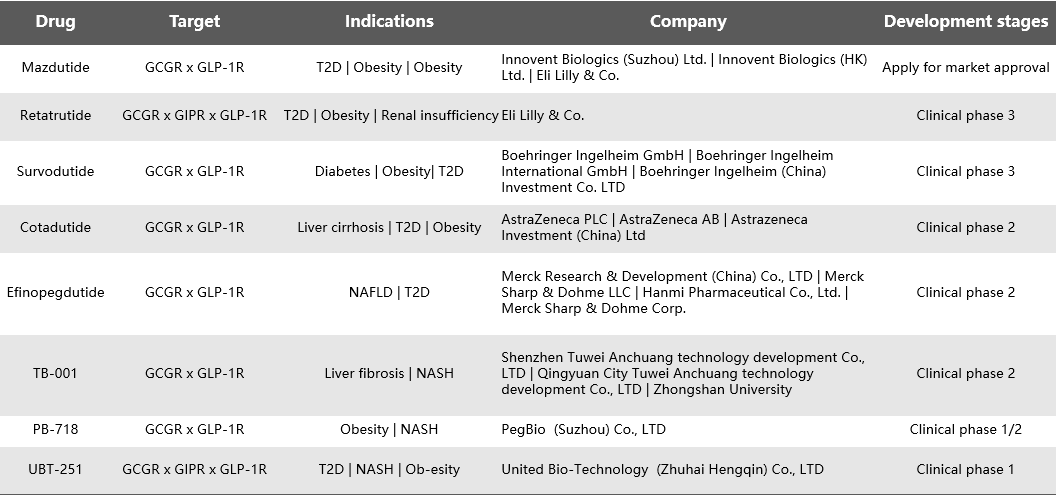
- Small molecule drugs
Currently, there are 2 small molecule drugs targeting GCGR in clinical development: PegBio’s GCGR-only agonist PB-722 and the Tianjin Instituteof Pharmaceutical Research’s GCGR x GLP-1R dual agonist TY-751.Bothof these drugs have had their clinical trial applications accepted for review.

- Recombinant protein & mAb
Currently, there are 3 fusion protein drugs and 1 monoclonal antibody targeting GCGR in clinical dev-elopment. Among the fusion proteins, the FGF21R x GCGR x GLP-1R triple-agonist DR10624 by Zhejiang Doer and the GCGR x GIPR x GLP-1R triple-agonist MWN-101 by Shanghai Minwei are in Phase 2 clinical trials. Hengrui’s GCGR x GLP-1R dual agonist SHR-1816 is in Phase 1 clinical trials. The monoclonal antibody drug Volagidemab is currently the only GCGR-targeting antagonist in clinical development. It is primarily used for the treatment of hypoglycemia and is currently in Phase 1 clinical trials.

- Hormone
Currently, there are 3 hormone-based drugs targeting GCGR in clinical d-evelopment, all of which are single-target therapies. Two of these have been approved for market: Novo Nordisk’s GCGR agonist, Glucagon, and Eli Lilly’s GCGR agonist, Glucagon. Additionally, Shanghai Domirui’s GCGR agonist,Glucagon (Domirui Biotechnology), is in Phase 1 clinical trials.

4.Products Related to the GCGR Target
Dima Biotechnology is a biotechnology company specializing in preclinical research products and services for druggable targets. We currently offer a range of ready-to-use products targeting GCGR, including recombinant ECD proteins, synthetic nanodisc full-length multi-transmembrane proteins, biosimilar reference antibodies, and biotin-labeled antibodies. Additionally, we provide comprehensive services such as custom protein/antibody services, antibody humanization, antibody affinity maturation, and stable cell line development. We have also established prevalidated B-cell seed library for the human GCGR target protein, allowing us to screen for lead monoclonal antibody molecules against this target protein within just 28 days. For more details, please contact us. (400-006-0995 / 18062749453)
| Product Type | SKU | Product Name |
| ECD protein | PME100722 | Human GCGR Protein, hFc Tag |
| Full-length multiple transmembrane protein | FLP100085 | Human GCGR full length protein-synthetic nanodisc |
| Biosimilar | BME100142 | Anti-GCGR(volagidemab biosimilar) mAb |
| Biotin-labeled antibody | BME100142B | Biotinylated Anti-GCGR(volagidemab biosimilar) mAb |
5.Progress in Research on GCGR Lead Molecules

1. Wewer Albrechtsen, N.J., et al., 100 years of glucagon and 100 more. Diabetologia, 2023. 66(8): p. 1378-1394.
2. Zhang, H., et al., Structure of the full-length glucagon class B G-protein-coupled receptor. Nature, 2017. 546(7657): p. 259-264.
3. Al-Massadi, O., et al., Glucagon Control on Food Intake and Energy Balance. Int J Mol Sci, 2019. 20(16).
4. Zhang, Y., et al., Glucagon Potentiates Insulin Secretion Via beta-Cell GCGR at Physiological Concentrations of Glucose. Cells, 2021. 10(9).
5. Jia, Y., et al., Role of Glucagon and Its Receptor in the Pathogenesis of Diabetes. Front Endocrinol (Lausanne), 2022. 13: p. 928016.
