In March 2024, AbbVie announced its decision to terminate the development of IMGC936 in collaboration with MacroGenics, citing that the clinical phase I trial results did not meet the expected safety and efficacy targets. IMGC936 was an antibody-drug conjugate (ADC) targeting ADAM9, initially developed through a partnership between ImmunoGen and MacroGenics, with each holding a 50% stake in the project. Last November, AbbVie acquired ImmunoGen for $10.1 billion. Despite the termination of IMGC936’s clinical development, MacroGenics has not given up on ADAM9 ADCs. The company is currently advancing another ADAM9-targeted ADC, MGC028, and plans to apply for approval to begin human trials by the end of this year. What makes ADAM9 so compelling that MacroGenics remains so committed to its development? And which other pharmaceutical companies are exploring this target?
1. The ADAM Family
A Disintegrin and Metalloprotease 9 (ADAM9), also known as MDC9 or Meltrin-γ, is a member of the A Disintegrin and Metalloprotease (ADAM) family, which belongs to the zinc metalloprotease superfamily. To date, approximately 40 ADAM family members have been identified in mammals, with 22 of them expressed in humans [1]. These include ADAM2, ADAM5, ADAM7, ADAM8, ADAM9, ADAM10, ADAM11, ADAM12, ADAM15, ADAM17, ADAM18, ADAM19, ADAM20, ADAM21 (ADAM31), ADAM22, ADAM23, ADAM27, ADAM28, ADAM29, ADAM30, ADAM32, and ADAM33 [2]. As shown in Figure 1, ADAM family members share a similar protein structure, which includes a signal peptide, prodomain, metalloprotease domain, disintegrin domain, cysteine-rich domain, transmembrane domain, and a cytoplasmic tail. Except for ADAM10 and ADAM17, other ADAMs possess an epidermal growth factor (EGF)-like domain between the cysteine-rich and transmembrane domains. The consensus sequence in the metalloprotease domain that determines ADAM protease activity is HEXGHXXGXXHD, where histidine binds to zinc ions and glutamic acid aids in catalysis [3]. It is noteworthy that not all ADAM family members have this consensus sequence in their metalloprotease domains; for instance, ADAM11, ADAM22, and ADAM23 are inactive ADAMs that primarily participate in cell-cell signaling through interactions with other proteins. Compared to the extracellular domains, the cytoplasmic domains of ADAMs are relatively short and contain various binding sites for intracellular signaling proteins [4].
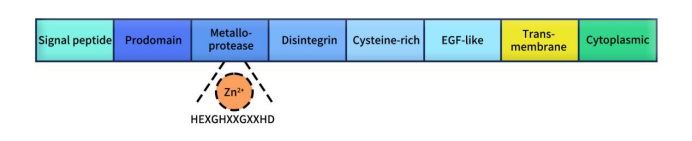
Figure 1. The typical structure of ADAMs [5]
2. Structure and Function of ADAM9
ADAM9 is present in both mice and humans and was first discovered in breast cancer in 1996. Its protein structure exhibits the typical ADAM architecture, comprising an N-terminal signal peptide (1-29 aa), a prodomain (30-205 aa), a metalloprotease domain (206-412 aa), a disintegrin domain (413-503 aa), a cysteine-rich domain (504-643 aa), an EGF-like domain (644-698 aa), a transmembrane domain (699-718 aa), and a cytoplasmic domain (719-819 aa). The N-terminal signal peptide directs ADAM9 to the cell surface, while the transmembrane domain anchors it in the cell membrane. ADAM9 is synthesized as an enzyme precursor, which is then processed by furin-like proprotein convertases (PCs). The metalloprotease domain contains a consensus sequence for zinc binding, which is crucial for its catalytic activity. The disintegrin domain interacts with various β1 integrins, thereby influencing adhesion to key extracellular matrix (ECM) components. The function of the EGF-like domain remains not fully understood. The cytoplasmic domain of ADAM9 contains four potential SH3 binding sites, which play a role in regulating its catalytic activity.
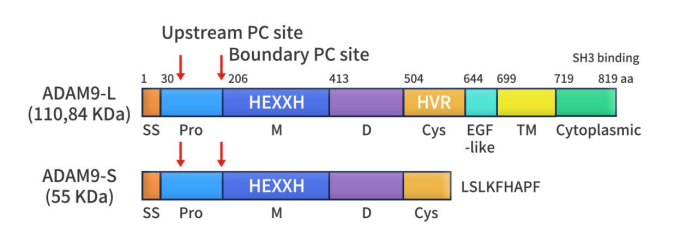
Figure 2. The characteristics of ADAM9 structure [6]
As shown in Figure 2, ADAM9 exists in two splice variants: the membrane-bound form (ADAM9-L) and the soluble form (ADAM9-S). ADAM9-S is a secreted protein that lacks the transmembrane and cytoplasmic domains due to the removal of exon 12 from ADAM9 mRNA. It also contains eight unique amino acids (LSLKFHAPF) not present in ADAM9-L. As a protease, ADAM9 can cleave various substrates at the cell surface, including cell adhesion molecules and growth factors, which are crucial for normal cell adhesion and migration. Additionally, ADAM9 is involved in cell migration and proliferation, promoting cell invasion and metastasis, and is associated with processes such as cell fusion and apoptosis. Disruptions in these functions are closely linked to the development of various diseases.
3. ADAM9 and Cancer
ADAM9 is widely expressed in various human tissues, including the lungs, colon, kidneys, vascular smooth muscle, nervous system, and reproductive system, with expression levels increasing under pathological conditions. It is present in several cell types, including monocytes, macrophages, neutrophils, keratinocytes, and fibroblasts. ADAM9 plays a significant role in the proliferation, invasion, metastasis, angiogenesis, and even immune evasion of various malignant tumors, such as liver cancer, breast cancer, lung cancer, gastric cancer, kidney cancer, and prostate cancer.
In lung cancer, ADAM9 is crucial for tumor progression and metastasis, with its overexpression associated with shorter overall survival. Metastasis is the leading cause of death in lung cancer, and nearly 50% of advanced lung cancer patients develop brain metastases. Shintani et al. first reported that ADAM9 overexpression enhances tumor cell adhesion to vascular endothelial cells, highlighting its importance in the metastatic process [7]. Research has shown that ADAM9 promotes lung cancer metastasis to the brain through a tissue-type plasminogen activator (tPA)-dependent mechanism that activates CDCP1 [8]. Fritzsche et al. found that overexpression of ADAM9 mRNA and protein is associated with lower recurrence-free survival in prostate cancer. Immunohistochemical analysis revealed that ADAM9 protein levels are elevated in over 60% of recurrent prostate tumors [9][10]. In breast cancer, ADAM9 expression is upregulated compared to normal tissue and contributes to disease progression by enhancing tumor extravasation and migration capabilities.
4. Clinical Research Progress on ADAM9-Targeted Therapies
Given ADAM9’s significant role in tumors and its differential expression, it has emerged as an attractive therapeutic target. Currently, all ADAM9-targeted drugs are antibody-drug conjugates (ADCs). However, only one, IMGC-936, has entered clinical trials, while AEX-6003 and MGC-028 are still in the preclinical stage.
- IMGC-936
IMGC-936 is an ADAM9-targeted ADC originally developed by MacroGenics in collaboration with ImmunoGen (now acquired by AbbVie). IMGC-936 consists of three components: a high-affinity humanized monoclonal antibody, a Maytansinoid microtubule inhibitor payload, and a stable tri-peptide linker, with a drug-to-antibody ratio (DAR) of 2. Preclinical data indicated that it can inhibit the progression of several tumor CDX models and demonstrated good tolerance in cynomolgus monkey toxicology studies. The drug has completed Phase I/II clinical trials for advanced malignant solid tumors (NCT04622774), which was a Phase I/II, first-in-human, open-label, dose-escalation, and expansion study. However, AbbVie has terminated its collaboration with MacroGenics on IMGC-936 because the trial results did not meet the expected safety and efficacy targets. It remains uncertain whether clinical development of IMGC-936 will proceed further.
- MGC-028
MGC-028 is a second-generation ADAM9-targeted ADC developed by MacroGenics. It utilizes a cleavable linker from Synaffix, a high-polarity spacer to enhance stability, and a toxin derived from exatecan, with a DAR of 4. At the 2024 AACR meeting, MacroGenics presented preclinical data showing that MGC-028 can inhibit the progression of several PDX tumor models. MacroGenics plans to apply for approval to initiate human trials by the end of this year.
- AEX-6003
AEX-6003 is an ADAM9 ADC drug developed by ImmunoGen. Its linker is the cleavable SPDB linker, and its toxin is Maytansinoid DM4. It is currently in the preclinical stage, with very little public information available about the drug. Ongoing monitoring is required.
5. DIMA Biotech’s ADAM9-Related Products Support Drug Development
DIMA Biotech is a biotechnology company specializing in preclinical development products and services for druggable targets. DIMA Biotech currently offers a full range of products and services related to the ADAM9 target. These include active proteins, reference antibodies, and flow cytometry validation monoclonal antibodies. Our services encompass customized antibody development for various species, antibody humanization, and affinity maturation.
To accelerate the development of ADAM9-based biologics, DIMA Biotech has also established an ADAM9 target single B-cell seed library, which can yield lead antibody molecules in as fast as 28 days. We have screened 42 lead ADAM9 molecules, including 22 human-monkey cross-reactive, 12 human-mouse cross-reactive, and 11 monkey-mouse cross-reactive molecules. Customers can obtain these molecules for functional evaluation the following day. For some molecules, we are also conducting ADC internalization activity and cytotoxicity validation. Specific data is available upon request.
- Recombinant Protein&Antibody&CDX SlideSet
| Product Type | Cat. No. | Product Name |
| Recombinant Protein | PME100901 | Human ADAM9 Protein, His Tag |
| PME100128 | Human ADAM9 Protein, hFc Tag | |
| PME-M100006 | Mouse ADAM9 Protein, His Tag | |
| PME-C100012 | Cynomolgus ADAM9 Protein, His Tag | |
| FC&IHC-validated Antibody | DME100192 | Anti-ADAM9 antibody(DM192); Rabbit mAb |
| DMC100832 | Anti-ADAM9 antibody(45G10), IgG1 Chimeric mAb | |
| Reference Antibody | BME100230 | Anti-ADAM9(izeltabart biosimilar) mAb |
| BME100064 | Anti-ADAM9 (biosimilar) mAb | |
| Biotin-labeled Antibody | BME100230B | Biotinylated Anti-ADAM9 (izeltabart biosimilar) mAb |
| BME100064B | Biotinylated Anti-ADAM9 (biosimilar) mAb | |
| DME100192B | Biotinylated Anti-ADAM9 antibody(DM192); Rabbit mAb | |
| CDX SlideSet | SLI100021 | M-NSG AGS DiSliceX™ SlideSet |
| SLI100014 | M-NSG SNU-5 DiSliceX™ SlideSet | |
| SLI100005 | Balb/C nu A431 DiSliceX™ SlideSet | |
| SLI100004 | Balb/C nu HuH7 DiSliceX™ SlideSet |
- Progress on ADAM9 Lead mAb Molecules

Reference:
[1]Weber S, Saftig P. Ectodomain shedding and ADAMs in development. Development. 2012 Oct;139(20):3693-709.
[2]Hsia, H. E., Tüshaus, J., Brummer, T., et al. Functions of ‘A disintegrin and metalloproteases (ADAMs)’ in the mammalian nervous system. Cellular and molecular life sciences : CMLS, 2019, 76(16), 3055–3081.
[3]Stocker W, Grams F, Baumann U, Reinemer P, Gomis-Ruth FX, McKay DB, Bode W. The metzincins—topological and sequential relations between the astacins, adamalysins, serralysins, and matrixins (collagenases) define a superfamily of zinc-peptidases. Protein Sci. 1995;4(5):823–840.
[4]Wolfsberg TG, White JM. ADAMs in fertilization and development. Dev Biol. 1996;180(2):389–401.
[5]Hsia, H. E., Tüshaus, J., Brummer, T., Zheng, Y., Scilabra, S. D., & Lichtenthaler, S. F. (2019). Functions of ‘A disintegrin and metalloproteases (ADAMs)’ in the mammalian nervous system. Cellular and molecular life sciences : CMLS, 76(16), 3055–3081.
[6]Chou, C. W., Huang, Y. K., Kuo, T. T., et al. An Overview of ADAM9: Structure, Activation, and Regulation in Human Diseases. International journal of molecular sciences, 2020, 21(20), 7790.
[7]Shintani Y., Higashiyama S., Ohta M., et al. Overexpression of ADAM9 in Non-Small Cell Lung Cancer Correlates with Brain Metastasis. Cancer Res. 2004;64:4190–4196.
[8]Lin C.Y., Chen H.J., Huang C.C., et al. ADAM9 promotes lung cancer metastases to brain by a plasminogen activator-based pathway. Cancer Res. 2014;74:5229–5243.
[9]ritzsche F.R., Jung M., Tölle A., et al. ADAM9 expression is a significant and independent prognostic marker of PSA relapse in prostate cancer. Eur. Urol. 2008;54:1097–1106.
Hua Y., Liang C., Miao C., et al. MicroRNA-126 inhibits proliferation and metastasis in prostate cancer via regulation of ADAM9. Oncol. Lett. 2018;15:9051–9060.
