At the 2024 ASCO Annual Meeting, one of the highlights was the MRG004A-001 study conducted by Professor Yu Xianjun’s team, marking a milestone in the exploration of escalating doses of MRG004A, an ADC drug targeting tissue factor TF/CD142. Carried out in the United States and China, the study’s findings are significant. Among 12 evaluated pancreatic cancer patients treated with MRG004A at 2.0 mg/kg, 33.3% (4/12) showed an objective response rate (ORR), while 83.3% (10/12) achieved disease control. These results highlight TF/CD142 as a promising therapeutic target and suggest potential breakthroughs in combating aggressive pancreatic cancer. What exactly is tissue factor TF? What is the current landscape of related targeted drugs?
1. Structure and Distribution of Tissue Factor (TF/CD142)
Tissue Factor (TF), also known as Coagulation factor III or CD142, is encoded by the F3 gene located on chromosome 1p21.3 in humans, spanning approximately 12.4 kb in length. The sequence encoding the TF protein consists of six exons. Exon 1 encodes the signal peptide and the translation start site; exons 2-5 encode the extracellular domain; and exon 6 encodes the transmembrane and cytoplasmic domains [1]. As shown in Figure 1, TF exists in three forms: full-length TF (flTF), alternatively spliced TF (asTF), and TF variant with alternative exon 1A (TF-A).
Human flTF is a single-pass transmembrane glycoprotein composed of 295 amino acids, featuring three domains: an extracellular domain (1-219 aa) comprising two fibronectin type III domains at the N-terminus, which complexes with factor VIIa in a membrane-dependent manner to significantly enhance the proteolytic activity towards its natural substrates factors IX, X, and VII; a transmembrane domain (220-242 aa) anchoring TF to the membrane; and a cytoplasmic domain (243-263 aa) involved in signal transduction [1].
asTF lacks a transmembrane domain and exists as a soluble form of TF generated through selective mRNA splicing, where exon 5 is skipped and exon 4 directly splices to exon 6 [2]. While not a potent procoagulant like flTF, asTF participates in non-hemostatic functions. In contrast to flTF, asTF can bind integrins independently of factor VII assistance. The TF-A variant is a new transcript of the TF gene generated through variable splicing of the first intron, resulting in an additional sequence called exon 1A derived from within the first intron, referred to as TF-A [3].
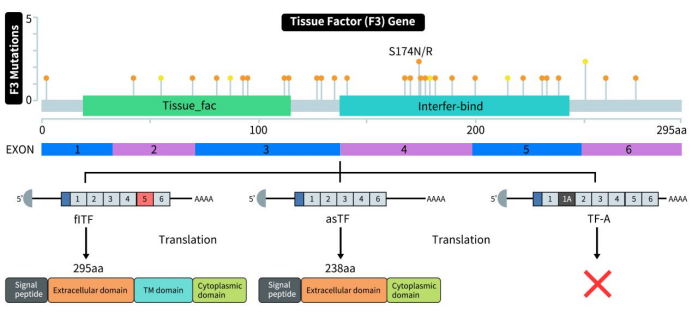
Figure 1. The structure of tissue factor (TF) [3]
Tissue Factor (TF) is constitutively expressed by vascular wall-associated cells, including vascular smooth muscle cells, adventitial fibroblasts, and pericytes. TF plays a role during embryonic development and is expressed in embryos before factors VII and VIIa. Typically, endothelial cells and macrophages do not express TF under normal physiological conditions to prevent unwanted clot formation. However, in pathological states like inflammation, atherosclerosis, and cancer, these cells can be induced to express TF. This aberrant expression is linked to the progression of these diseases, as TF is involved in various cellular processes beyond coagulation, including cell signaling pathways that promote cancer cell proliferation, survival, metastasis, angiogenesis, and venous thromboembolism (VTE).
2. Multifaceted Functions of Tissue Factor (TF/CD142)
Tissue Factor (TF) is renowned for its pivotal role as the primary initiator of the coagulation cascade, safeguarding vascular integrity upon injury. Under normal conditions, TF, upon exposure to blood, associates with phospholipid membranes and calcium ions to form a complex with activated factor VII (FVIIa). This TF-FVIIa complex undergoes conformational changes, augmenting its enzymatic activity to catalyze the conversion of factor X (FX) to its activated form, FXa. The resultant FXa generation is a critical step that leads to thrombin production, platelet activation, and fibrin clot formation, ultimately restoring vascular integrity.
Beyond its classical hemostatic function, TF’s involvement in angiogenesis has garnered significant attention. The TF-FVIIa complex is known to cleave protease-activated receptor 2 (PAR2), which results in the phosphorylation of TF’s cytoplasmic domain. This phosphorylation event modulates the signaling pathways mediated by PAR2, effectively lifting the negative regulation and promoting angiogenesis. This non-hemostatic role of TF, particularly its influence on angiogenesis, underscores its importance in both physiological processes and pathological conditions, including cancer progression [4].
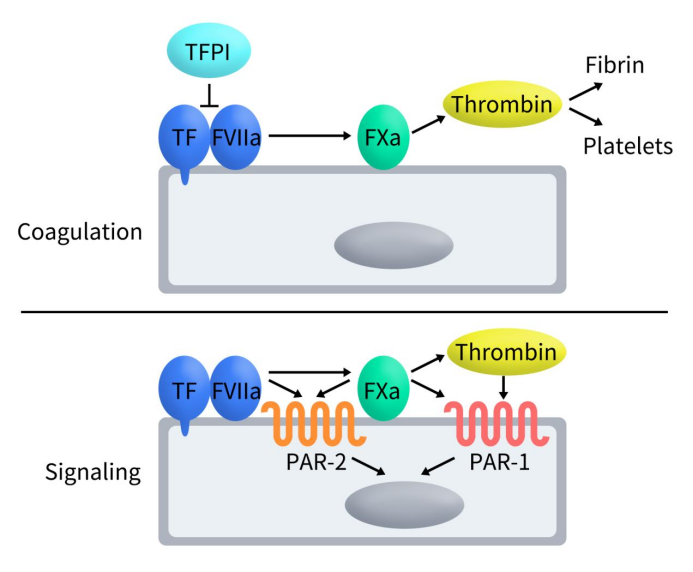
Figure 2. The role and mechanism of TF [4]
3. Tissue Factor (TF/CD142) and Its Pivotal Role in Cancer Dynamics
Tissue Factor (TF), prominently expressed on the surface of numerous cancer cells, is a key player in the intricate ballet of cancer progression, invasion, metastasis, and angiogenesis. As shown in the diagram below, TF can activate the JAK2-STAT5 pathway, leading to uncontrolled cancer cell growth and inhibiting apoptosis. It can also activate receptor tyrosine kinases (RTKs) to promote tumor cell growth. TF increases VEGF production while inhibiting TSP, thereby enhancing tumor angiogenesis and metastasis. Additionally, TF activation of rac1 further activates PKA, regulating MAPK to promote cancer cell growth and metastasis. TF can also activate PAR2, which enhances angiogenesis, invasion, and metastasis of cancer cells through various mechanisms. PAR2 promotes angiogenesis and cancer cell invasion and metastasis by increasing VEGF, bEGF, IL8, and βTGF levels. Moreover, PAR2 stabilizes β-catenin, leading to tumor cell invasion. Furthermore, PAR2 activates the MAPK and ERK1/2 pathways to increase β-arrestin, which phosphorylates actin-depolymerizing factor, enhancing the polymerization of actin filaments at the edges of invading cells and thereby promoting cancer cell invasion and metastasis.
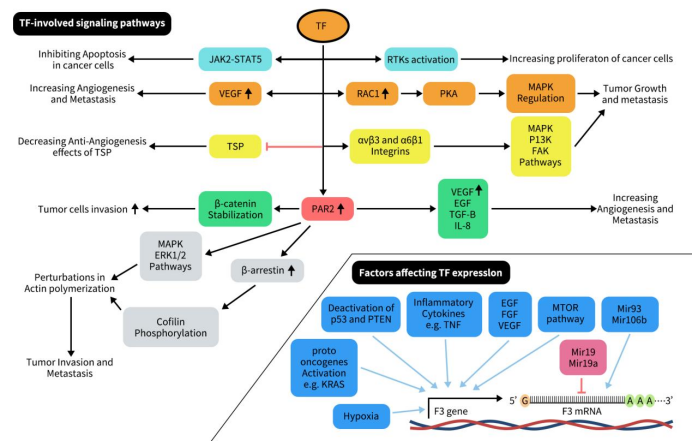
Figure 3. Overview of factors affecting F3 gene and TF-involved signaling pathways progressing cancer [3]
4. Advances in Tissue Factor (TF/CD142) Targeted Therapy
The unique expression profile of Tissue Factor (TF) makes it an ideal target for delivering drugs selectively to tumor cells without targeting normal tissue cells. As a prime target for Antibody-Drug Conjugate (ADC) therapies, TF has catalyzed the development of several promising drugs. Some, like Seagen and Genmab’s Tisotumab Vedotin, have already received FDA approval for the treatment of cervical cancer, marking the first green light for a TF-directed therapeutic. The promising results of MRG004A further underscore the potential of TF-targeted therapies in managing not just pancreatic cancer but potentially other solid tumors with high TF expression. XNW-28012 developed by Shanghai Sinovent Biopharmaceutical is currently in Phase I clinical trials and XB002 developed by Iconic Therapeutics is currently in Phase I/II clinical trials.
- Tisotumab vedotin
Tisotumab vedotin, marketed as Tivdak, is an ADC drug developed jointly by Seagen and GenMab, and it represents the first ADC drug targeting Tissue Factor (TF). Tisotumab vedotin consists of three components: the monoclonal antibody Tisotumab targeting TF, a cleavable peptide linker, and the cytotoxin monomethyl auristatin E (MMAE) with a drug-to-antibody ratio (DAR) of 4. When Tisotumab vedotin interacts with TF-positive tumor cells, it undergoes internalization and subsequent lysosomal degradation, releasing MMAE inside the tumor cells. MMAE is a potent anti-mitotic agent that binds to tubulin and disrupts microtubule assembly, inducing cell cycle arrest at the G2/M phase and thereby triggering apoptosis. Additionally, MMAE can be released extracellularly in the tumor microenvironment, causing bystander cell death (bystander effect). [5].
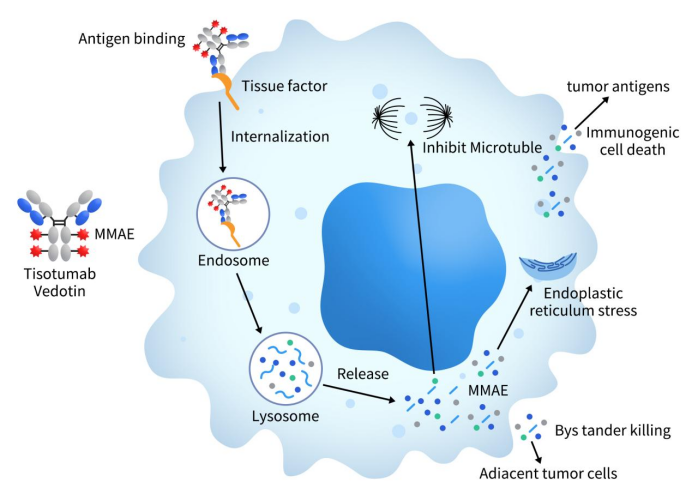
Figure 4. The mechanism of tisotumab vedotin [5]
In September 20, 2021, Tisotumab vedotin received accelerated approval from the FDA for the indication of second-line treatment of recurrent or metastatic cervical cancer in adult patients. The approval was based on pivotal trial results from ENGOT-cx6/GOG-3023/innovaTV 204. This trial demonstrated an objective response rate (ORR) of 24% in women with recurrent or metastatic cervical cancer who had disease progression after platinum-based therapy, with a notable duration of response [5]. In September 2022, ReDing Pharmaceuticals entered into a partnership agreement with Seagen to obtain exclusive rights for the development and commercialization of Tisotumab vedotin in mainland China, Hong Kong, Macau, and Taiwan.
- XNW-28012
XNW-28012 is an innovative ADC drug that targets tissue factor (TF), developed by Shanghai Sinovent Biopharmaceutical Co., Ltd. It’s currently in the clinical trial phase, with a focus on treating advanced malignant solid neoplasms. The small molecule component of XNW-28012 is based on the YiLink Bio TMALIN platform, which is known for its dual cleavage mechanisms that operate both extracellularly in the tumor microenvironment and intracellularly in lysosomes. The payload YL0014 used in XNW-28012 is a topoisomerase I inhibitor, which exhibits higher cellular potency than some other payloads used in ADCs. As for the clinical trials, XNW-28012 has been approved to initiate studies focusing on its safety, tolerability, pharmacokinetics, and preliminary anti-tumor activity in patients with advanced solid tumors. The Phase I/II clinical study began on November 29, 2023.
- MRG004A
MRG004A is a cutting-edge ADC developed by Leap Therapeutics, targeting tissue factor (TF) and employing Synaffix’s site-specific conjugation technology. The drug has been recognized by the FDA with an orphan drug designation for pancreatic cancer treatment in December 2023, and subsequently, it received Fast Track designation in March 2024.
The ongoing Phase I/II clinical trials of MRG004A, identified as NCT04843709, are being conducted in both the United States and China. These trials aim to evaluate the safety, efficacy, pharmacokinetics, and immunogenicity of MRG004A in patients with tissue factor-positive advanced or metastatic solid tumors. The study consists of two parts: a dose escalation study to determine the maximum tolerated dose (MTD) and recommended phase II dose (RP2D), and a disease-specific multi-cohort dose expansion study to further assess the efficacy and safety of MRG004A at the confirmed RP2D. At the ASCO 2024 conference, intermediate clinical data from these trials was presented.
- XB002
XB002 is a next-generation ADC that targets tissue factor (TF), and it’s a product of collaboration between Zymeworks and Exelixis. The drug leverages Zymeworks’ proprietary ZymeLink™ technology, which includes a linker-payload designed for an improved therapeutic index and favorable safety profile. The payload used in XB002 is an auristatin-based cytotoxic agent, which is a derivative of MMAE.
The development rights for XB002 were acquired by Exelixis from Iconic Therapeutics in 2019, and since then, Exelixis has been responsible for the clinical development, commercialization, and manufacturing of XB002. The Phase I clinical trials, identified as NCT04925284, are currently underway. These trials evaluate the safety, tolerability, pharmacokinetics, and preliminary antitumor activity of XB002 in patients with advanced solid tumors.
Recent updates from the JEWEL-101 study have shown that XB002 was well-tolerated at multiple dose levels, with low-grade ocular toxicity and no bleeding events or treatment-related peripheral neuropathy reported. The study is now progressing to the cohort expansion stage, where the safety and preliminary antitumor activity of XB002 will be further evaluated in patients with specific types of advanced solid tumors, including metastatic castration-resistant prostate cancer.
5. DIMA Biotech: Advancing TF Biotherapy Development
DIMA Biotech is a biotechnology company specializing in preclinical development products and services focused on druggable targets. The company offers a full range of products and services targeting TF. DIMA’s products include active proteins, reference antibodies, flow cytometry validated monoclonal antibodies, and stable cell lines. DIMA Biotech provides services such as custom antibody production across various species, antibody humanization, and affinity maturation services. Additionally, to accelerate the development of TF-based biopharmaceuticals, DIMA Biotech has developed a single B cell seed library specific to TF, enabling clients to obtain lead antibody molecules in as little as 28 days.
- Recombinant Protein&Antibody&Stable Cell Line
| Product Type | Cat. No. | Product Name |
| Recombinant Protein | PME100751 | Human CD142 Protein, hFc Tag |
| FC-validated Antibody | DMC100463 | Anti-CD142 antibody(DMC463); IgG1 Chimeric mAb |
| Reference Antibody | BME100124 | Anti-CD142(tisotumab biosimilar) mAb |
| Biotin-labeled Antibody | BME100124B | Biotinylated Anti-CD142(tisotumab biosimilar) mAb |
| DMC100463B | Biotinylated Anti-CD142 antibody(DMC463); IgG1 Chimeric mAb | |
| Stable Cell Line | CEL100049 | Hu_CD142 K562 Cell Line |
- Progress on TF/CD142 Lead mAb Molecules

References:
[1]Butenas, Saulius. Tissue factor structure and function. Scientifica vol. 2012 (2012): 964862.
[2]Bogdanov VY, Balasubramanian V, Hathcock J, et al. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat Med. 2003 Apr;9(4):458-62.
[3]Ahmadi, S. E., Shabannezhad, A., Kahrizi, A., et al. Tissue factor (coagulation factor III): a potential double-edge molecule to be targeted and re-targeted toward cancer. Biomarker research, 2023, 11(1), 60.
[4]Mackman, N., & Taubman, M. Tissue factor: past, present, and future. Arteriosclerosis, thrombosis, and vascular biology, 2009, 29(12), 1986–1988.
Agostinelli, V., Musacchio, L., Camarda, F., et al. Therapeutic Potential of Tisotumab Vedotin in the Treatment of Recurrent or Metastatic Cervical Cancer: A Short Report on the Emerging Data. Cancer management and research, 2023, 15, 1063–1072.
