In recent years, the rapid development of AI-driven drug discovery (AI-based drug discovery) and Cryo-EM (cryo-electron microscopy) has gradually unveiled the mysteries of many previously considered “undruggable” protein targets. Among them, membrane proteins, which are crucial targets for drug development, are now seeing unprecedented breakthroughs under the dual influence of AI and Cryo-EM technologies.
However, membrane protein research has always faced significant challenges, such as difficulty in stable extraction, tendency to denature, and challenges in structural analysis. These issues have made it difficult to obtain high-resolution structures using traditional research methods like X-ray crystallography. Fortunately, the advent of Nanodisc technology has provided a solution that offers a more physiologically relevant environment for membrane protein research, particularly showcasing its great advantages in Cryo-EM studies.
In this article, we will explore the relationship between membrane proteins, AI-driven drug discovery, and Cryo-EM, as well as highlight the unique advantages and potential applications of Nanodisc technology in membrane protein research.
1. Membrane Proteins, AI Drug Discovery, and Cryo-EM: Why Are They Emerging as Hot Research Areas?
In recent years, membrane proteins, AI-driven drug discovery, and cryo-electron microscopy (Cryo-EM) have become trending topics in biomedical research because they collectively drive breakthroughs in drug development, especially in the fields of targeted therapy and precision medicine. Here are some key reasons why these areas have gained significant attention:
1.1 The Critical Role of Membrane Proteins in Drug Development
Membrane proteins are widely distributed on cell membranes and perform vital physiological functions, such as substance transport, signal transduction, and cell communication. Their essential roles in maintaining normal bodily functions make them critical targets in drug development. Studies show that approximately 60% of FDA-approved drugs target membrane proteins, highlighting their importance in pharmaceutical research. Below are some major classes of membrane proteins and their applications in drug development:
- G Protein-Coupled Receptors (GPCRs):
GPCRs are among the most common drug targets, with around 30% of prescription drugs acting on them. Common GPCR-targeting drugs include β-blockers (used to treat hypertension and heart failure) and opioid analgesics (used to relieve severe pain). These drugs work by binding to GPCRs and modulating signaling pathways to exert therapeutic effects.
- Ion Channels:
Ion channels play a crucial role in the transmission of nerve signals. Many antiepileptic and pain-relieving drugs function by regulating ion channel activity. For example, antiepileptic drugs often inhibit sodium channels to slow down nerve impulse transmission, thereby controlling seizures.
- ATP-Binding Cassette (ABC) Transporters:
ABC transporters are a key class of membrane proteins involved in transmembrane transport of various substances, including drug efflux. P-glycoprotein (P-gp), a major ABC transporter, is closely linked to cancer drug resistance. Many cancer therapies aim to target ABC transporters to overcome resistance and improve treatment efficacy.
Despite their critical potential in drug development, membrane proteins pose significant challenges due to their complex and unstable nature. They are often difficult to extract, purify, and stabilize, and are prone to denaturation during experiments. These issues make it difficult for traditional structural analysis methods like X-ray crystallography to capture high-resolution structural data. As a result, progress in membrane protein research has traditionally been slow, despite their tremendous therapeutic potential.
1.2 The Role of AI in Membrane Protein Drug Development
In recent years, artificial intelligence (AI) has made significant strides in areas such as protein structure prediction, molecular docking, and drug screening. Its application in membrane protein research is driving breakthroughs in both drug development and biological studies. Specifically, AI contributes to membrane protein research in the following ways:
- Membrane Protein Structure Prediction:
Due to their complex structures, membrane proteins have long posed challenges for structural analysis. AI technologies—particularly AlphaFold2—have made tremendous advances in protein structure prediction. AlphaFold2 utilizes deep learning models to accurately and efficiently predict the 3D structures of many membrane proteins. This breakthrough greatly improves research efficiency and provides more accurate structural data for drug targeting, thereby accelerating the discovery of membrane protein-targeted drugs.
- AI-Powered Molecular Docking for Drug Screening:
AI can be integrated with molecular docking techniques to analyze interactions between membrane proteins and ligands. By performing virtual screening of large molecular libraries, AI can predict potential drug candidates and improve the accuracy of hit identification. This approach significantly reduces the cost of experimental screening and accelerates the drug development process. In the case of complex targets like membrane proteins, AI effectively compensates for the limitations of traditional methods.
- Optimizing Membrane Protein Stability to Improve Cryo-EM Success Rates:
The instability of membrane proteins has long hindered Cryo-EM (cryo-electron microscopy) research. AI can analyze conformational changes and predict membrane protein stability under various conditions. By combining AI’s predictions of dynamic structural states with Cryo-EM techniques, researchers can obtain more accurate structural information. This enhances the overall success rate of structural determination and makes Cryo-EM more efficient in membrane protein research, paving the way for breakthroughs in structural biology and drug discovery.
Through these applications, AI not only accelerates the pace of membrane protein research but also provides more precise and efficient tools for drug development, significantly advancing the discovery of new therapeutics targeting membrane proteins.
1.3 Cryo-EM: A Revolutionary Technique for Membrane Protein Structure Analysis
Cryo-electron microscopy (Cryo-EM) has become one of the most important technologies in the life sciences in recent years. In 2017, three scientists were awarded the Nobel Prize in Chemistry for their contributions to Cryo-EM, marking a major breakthrough in structural biology. Cryo-EM works by rapidly freezing biological samples at liquid nitrogen temperatures—typically using liquid ethane—into vitreous ice, which effectively preserves the protein’s native conformation. This technique has two key innovations: first, it eliminates the need for crystallization, and second, it allows researchers to capture multiple conformations, including transient and dynamic states—features that are especially critical for studying complex structures like membrane proteins.
Advantages of Cryo-EM in Membrane Protein Analysis

- No Crystallization Required:
Membrane proteins are notoriously difficult to crystallize, which limits the effectiveness of traditional methods such as X-ray crystallography. Cryo-EM circumvents this issue by eliminating the need for crystallization altogether.
- Suitable for Large Macromolecular Complexes:
Cryo-EM is particularly well-suited for resolving large macromolecular assemblies. This is especially important for studying membrane proteins, which often function as complexes with other biomolecules such as ligands or cofactors. Cryo-EM can provide high-resolution structures of these entire assemblies.
- Captures Dynamic Conformations:
Membrane proteins possess complex three-dimensional structures and undergo significant conformational changes. Traditional techniques struggle to capture these dynamic transitions. Cryo-EM, however, can visualize proteins in multiple conformational states, offering detailed insights into how membrane proteins interact with ligands or other molecules.
In recent years, Cryo-EM has successfully resolved high-resolution structures of numerous membrane proteins, offering critical guidance for drug discovery and development.
Table 1. Examples of How AI + Cryo-EM Accelerate Membrane Protein Drug Development
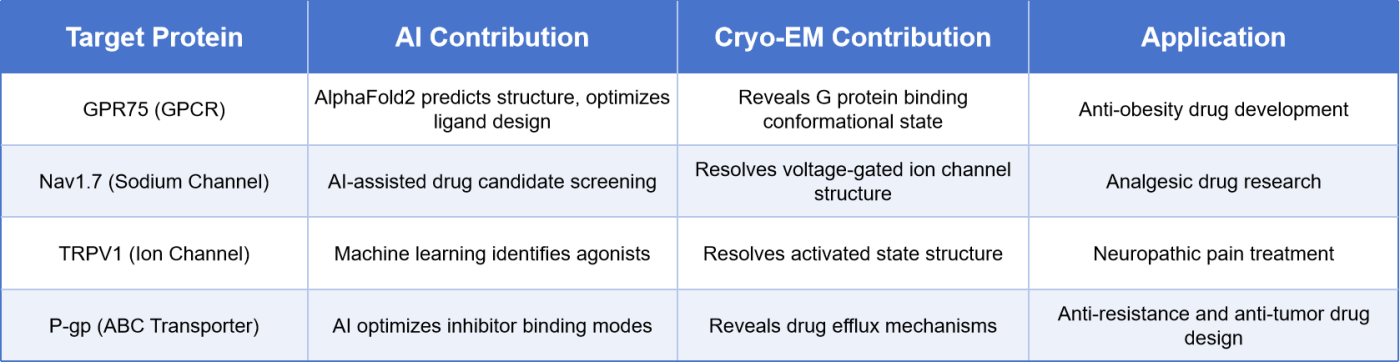
The groundbreaking advancements of Cryo-EM have not only opened up new possibilities for membrane protein research but also created promising avenues for drug development. By resolving the three-dimensional structures of membrane proteins, Cryo-EM enables researchers to precisely design drug molecules that target these proteins, facilitating personalized therapies and efficient drug screening. Furthermore, the high-resolution data provided by Cryo-EM offers detailed structural insights that significantly enhance AI-assisted drug discovery, boosting progress in virtual screening and molecular docking technologies.
Cryo-EM holds unparalleled advantages in membrane protein structural analysis, particularly in its ability to bypass crystallization, accommodate large macromolecular complexes, and capture dynamic conformations. As the technology continues to evolve, Cryo-EM is poised to remain a key tool in structural biology and pharmaceutical research, playing a central role in membrane protein studies and novel drug discovery.
2. Nanodisc: An Ideal Tool for Studying Membrane Proteins with Cryo-EM
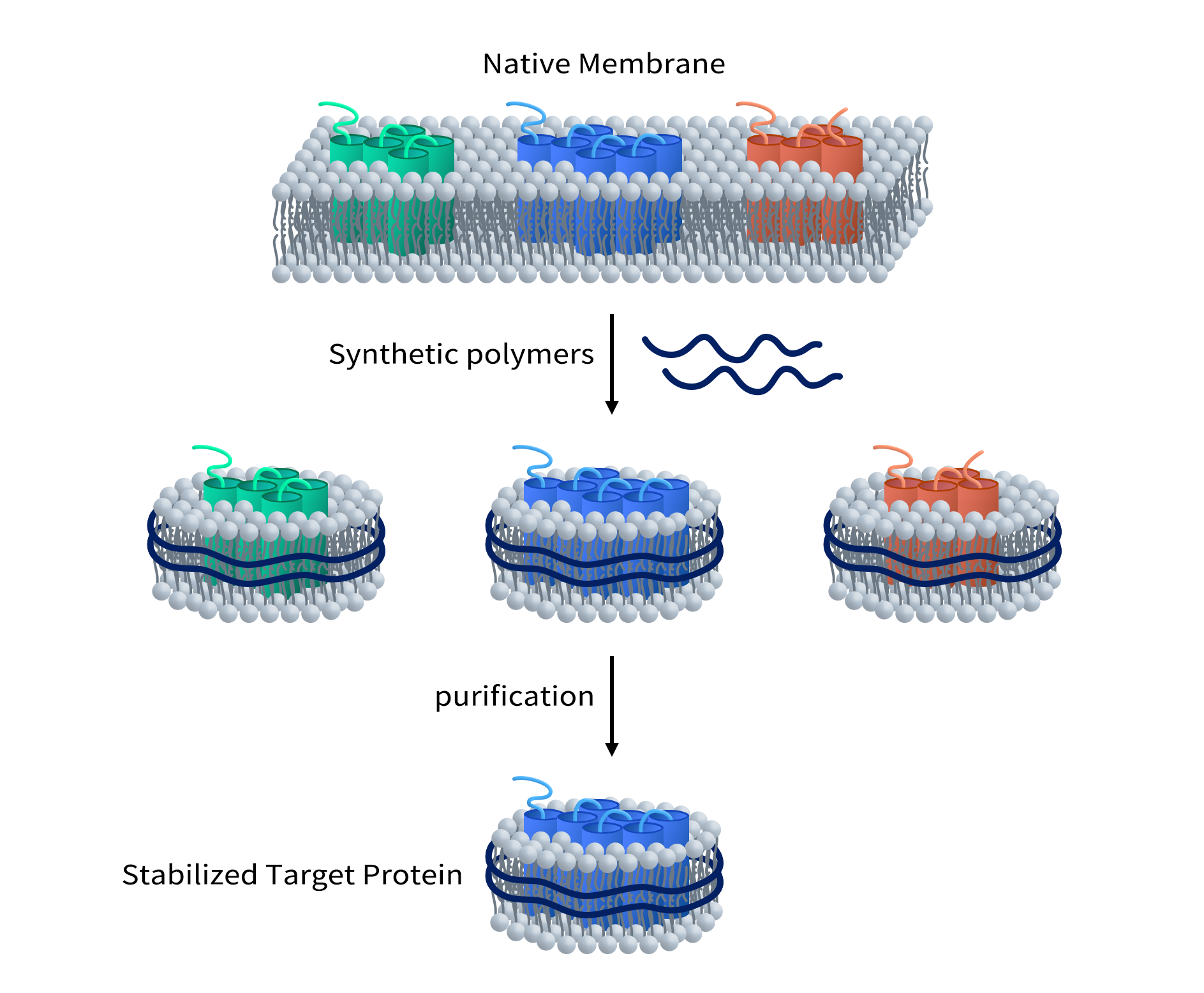
Although Cryo-EM (cryo-electron microscopy) has significantly advanced the study of membrane proteins, the stability of membrane proteins remains a major challenge. Traditional methods for extracting membrane proteins usually rely on detergents to dissolve the cell membrane and release the proteins. However, while detergents can extract membrane proteins, they can also disrupt the natural structure of these proteins, leading to a loss of function or instability in solution. This instability limits further research on membrane proteins, especially in structural analysis and drug development.
Nanodisc technology, as an innovative method for stabilizing membrane proteins, provides an alternative to detergents, allowing membrane proteins to remain stable in a lipid environment that closely mimics physiological conditions. Nanodisc technology not only overcomes the damaging effects of detergents on membrane protein structure and function but also offers an ideal solution for Cryo-EM studies.
A nanodisc is a nanoscale complex composed of lipid molecules and membrane proteins. By stabilizing membrane proteins within nanodiscs, researchers can obtain high-resolution three-dimensional structures of membrane proteins using Cryo-EM. These structural data provide valuable information for functional studies of membrane proteins, drug target discovery, and new drug development. With the combination of nanodisc technology and Cryo-EM, structural analysis of membrane proteins will become more efficient and accurate, thus providing stronger support for the treatment of membrane protein-related diseases and drug development.
The Advantages of Nanodisc Technology in Membrane Protein Research
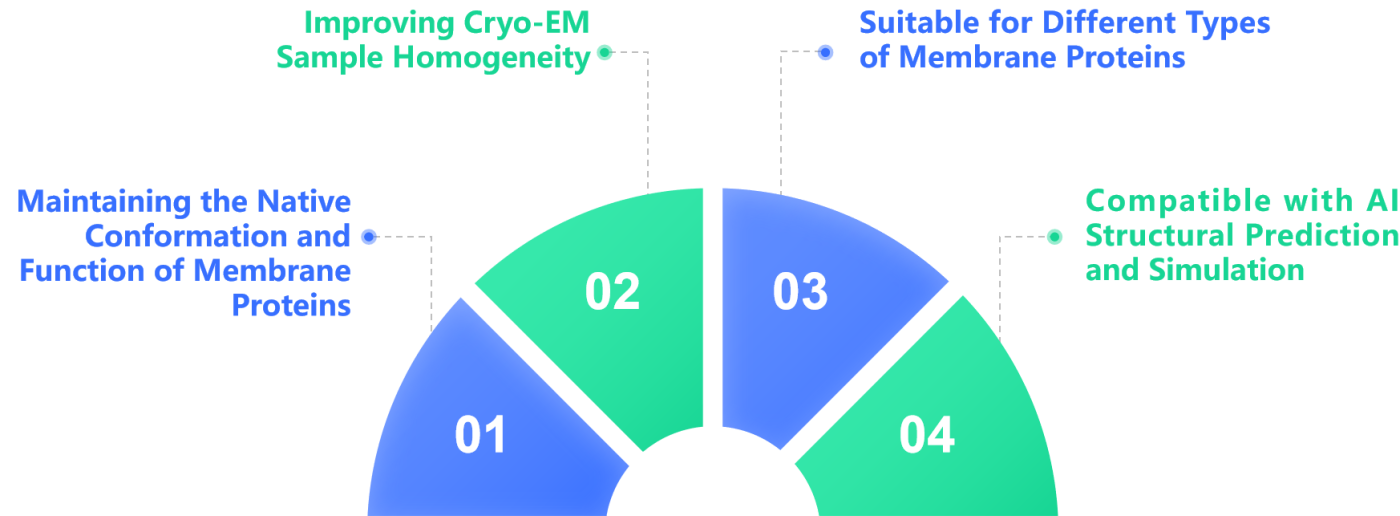
- Improving Cryo-EM Sample Homogeneity:
Traditional membrane protein samples often suffer from aggregation issues, which can negatively impact the quality of Cryo-EM imaging. In contrast, Nanodiscs provide highly homogeneous samples, thereby improving the quality of the data.
- Maintaining the Native Conformation and Function of Membrane Proteins:
Nanodiscs are composed of lipid bilayers and membrane proteins, simulating the natural cell membrane environment, which helps maintain the activity of the membrane proteins.
- Suitable for Different Types of Membrane Proteins:
Nanodiscs can be used to stabilize a variety of membrane proteins, including GPCRs, ion channels, transporters, and others, enhancing the success rate of structural analysis.
- Compatible with AI Structural Prediction and Simulation:
AI, combined with the stable structural data provided by Nanodiscs, can further optimize the drug screening process for membrane proteins.
3. DIMA Nanodisc Products
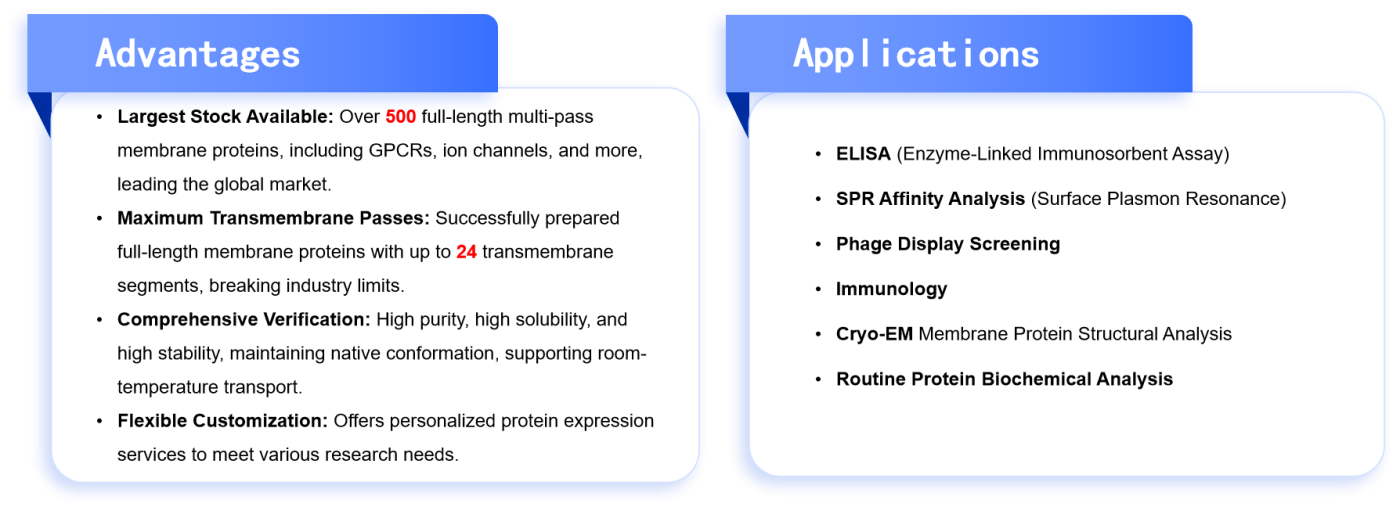
To better support membrane protein research, Dima offers a range of high-quality Nanodisc-related products, suitable for Cryo-EM studies and AI-assisted drug development. Dima’s Nanodisc products offer significant advantages in terms of membrane protein stability, construction flexibility, and broad applicability, making them the ideal choice for researchers and drug developers in membrane protein studies.
Table 2. Advantages and Related Applications of Dima Nanodisc Products
3.1 Data Presentation
SDS-PAGE

SEC-HPLC Purity Detection
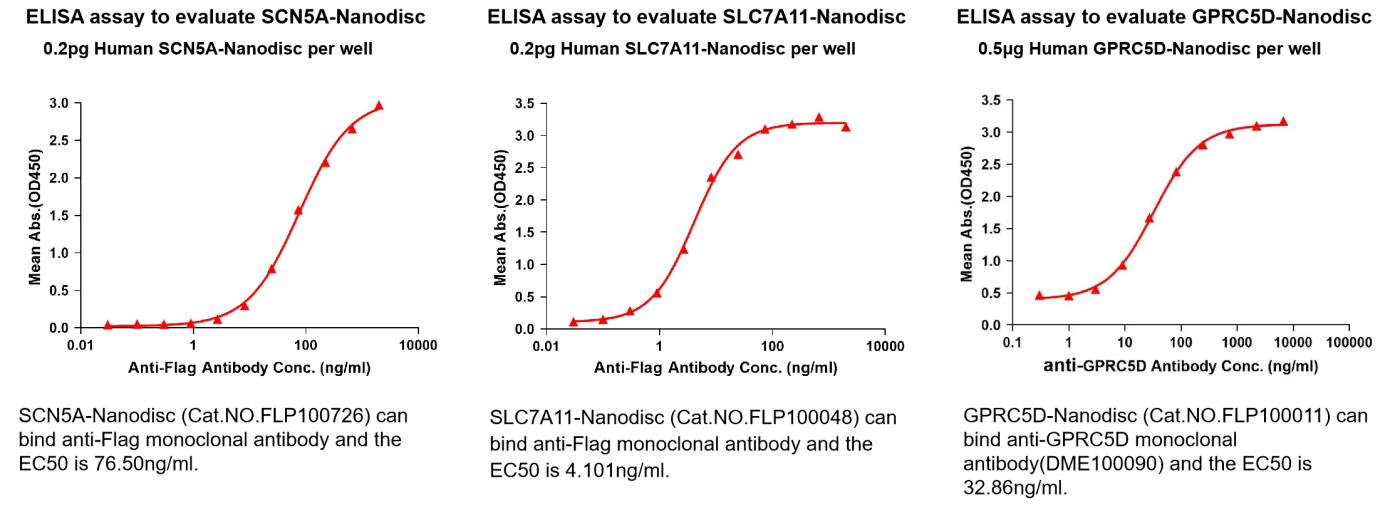
ELISA Analysis
SPR Affinity Detection
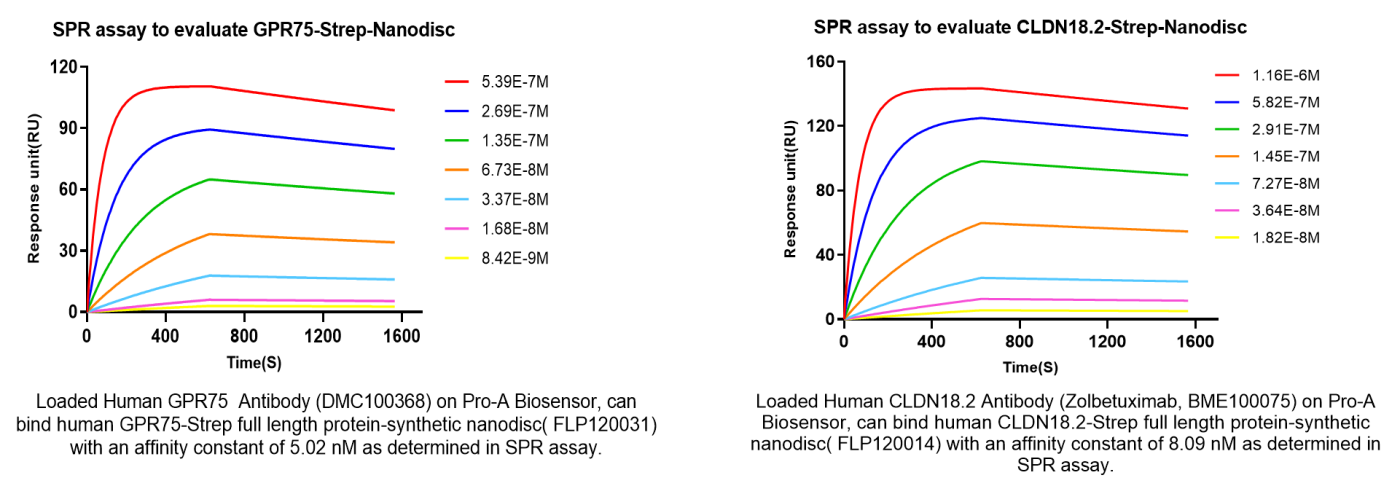
3.2 Partial Product List
Table 3. Partial Nanodisc Product List
| sku | Product name |
| FLP100011 | Human GPRC5D full length protein-synthetic nanodisc |
| FLP100013 | Human SSTR2 full length protein-synthetic nanodisc |
| FLP100020 | Human ADORA2A full length protein-synthetic nanodisc |
| FLP100023 | Human CB1 full length protein-synthetic nanodisc |
| FLP100024 | Human CCR4 full length protein-synthetic nanodisc |
| FLP100031 | Human GPR75 full length protein-synthetic nanodisc |
| FLP100036 | Human F2RL1 full length protein-synthetic nanodisc |
| FLP100037 | Human CCR8 full length protein-synthetic nanodisc |
| FLP100045 | Human TSHR full length protein-synthetic nanodisc |
| FLP100047 | Human FSHR full length protein-synthetic nanodisc |
| FLP100052 | Human FZD10 full length protein-synthetic nanodisc |
| FLP100053 | Human CXCR3 full length protein-synthetic nanodisc |
| FLP100054 | Human GPR55 full length protein-synthetic nanodisc |
| FLP100059 | Human CCR6 full length protein-synthetic nanodisc |
| FLP100060 | Human CCR7 full length protein-synthetic nanodisc |
| FLP100061 | Human CCR9 full length protein-synthetic nanodisc |
| FLP100062 | Human GPR87 full length protein-synthetic nanodisc |
| FLP100066 | Human CXCR2 full length protein-synthetic nanodisc |
| FLP100067 | Human CXCR5 full length protein-synthetic nanodisc |
| FLP100069 | Human GPR77 full length protein-synthetic nanodisc |
| FLP100072 | Human LGR4 full length protein-synthetic nanodisc |
| FLP100073 | Human LGR5 full length protein-synthetic nanodisc |
| FLP100074 | Human CXCR4 full length protein-synthetic nanodisc |
| FLP100075 | Human CCR3 full length protein-synthetic nanodisc |
| FLP100085 | Human GCGR full length protein-synthetic nanodisc |
| FLP100086 | Human C5AR1 full length protein-synthetic nanodisc |
| FLP100090 | Human ADGRE2 full length protein-synthetic nanodisc |
| FLP100091 | Human CXCR1 full length protein-synthetic nanodisc |
| FLP100092 | Human GNRHR full length protein-synthetic nanodisc |
| FLP100093 | Human ADORA2B full length protein-synthetic nanodisc |
| FLP100094 | Human CCR1 full length protein-synthetic nanodisc |
| FLP100095 | Human CXCR7 full length protein-synthetic nanodisc |
| FLP100096 | Human PTGER2 full length protein-synthetic nanodisc |
| FLP100097 | Human PTGER4 full length protein-synthetic nanodisc |
| FLP100099 | Human HCRTR1 full length protein-synthetic nanodisc |
| FLP100100 | Human CCR5 full length protein-synthetic nanodisc |
| FLP100102 | Human PROKR1 full length protein-synthetic nanodisc |
| FLP100103 | Human GPR65 full length protein-synthetic nanodisc |
| FLP100104 | Human ADGRG1 full length protein-synthetic nanodisc |
| FLP100105 | Human MRGPRX2 full length protein-synthetic nanodisc |
| FLP100107 | Human ADGRG2 full length protein-synthetic nanodisc |
| FLP100108 | Human GPR20 full length protein-synthetic nanodisc |
| FLP100111 | Human CHRM2 full length protein-synthetic nanodisc |
| FLP100113 | Human GLP2R full length protein-synthetic nanodisc |
| FLP100114 | Human F2RL3 full length protein-synthetic nanodisc |
| FLP100115 | Human CX3CR1 full length protein-synthetic nanodisc |
| FLP100116 | Human GPR132 full length protein-synthetic nanodisc |
| FLP100117 | Human ADGRE5 full length protein-synthetic nanodisc |
| FLP100118 | Human GRPR full length protein-synthetic nanodisc |
| FLP100119 | Human GRM7 full length protein-synthetic nanodisc |
| FLP100120 | Human XCR1 full length protein-synthetic nanodisc |
| FLP100121 | Human GLP1R full length protein-synthetic nanodisc |
| FLP100122 | Human MC4R full length protein-synthetic nanodisc |
| FLP100123 | Human BDKRB2 full length protein-synthetic nanodisc |
| FLP100124 | Human CXCR6 full length protein-synthetic nanodisc |
| FLP100125 | Human PTGDR2 full length protein-synthetic nanodisc |
| FLP100126 | Human CMKLR1 full length protein-synthetic nanodisc |
| FLP100129 | Human GPR84 full length protein-synthetic nanodisc |
| FLP100130 | Human GIPR full length protein-synthetic nanodisc |
| FLP100131 | Human NTSR1 full length protein-synthetic nanodisc |
| FLP100132 | Human APLNR full length protein-synthetic nanodisc |
| FLP100134 | Human FFAR1 full length protein-synthetic nanodisc |
| FLP100135 | Human GRM2 full length protein-synthetic nanodisc |
| FLP100136 | Human UTS2R full length protein-synthetic nanodisc |
| FLP100138 | Human CB2 full length protein-synthetic nanodisc |
| FLP100139 | Human FZD4 full length protein-synthetic nanodisc |
| FLP100145 | Human CGRPR-RAMP1 full length protein-synthetic nanodisc |
| FLP100172 | Human 5HT7R full length protein-synthetic nanodisc |
| FLP100175 | Human ACKR1 full length protein-synthetic nanodisc |
| FLP100186 | Human ADRA2A full length protein-synthetic nanodisc |
| FLP100216 | Human CALCR full length protein-synthetic nanodisc |
| FLP100219 | Human CCR10 full length protein-synthetic nanodisc |
| FLP100230 | Human DRD2 full length protein-synthetic nanodisc |
| FLP100297 | Human GPR21 full length protein-synthetic nanodisc |
| FLP100308 | Human GPR39 full length protein-synthetic nanodisc |
| FLP100333 | Human KISSR full length protein-synthetic nanodisc |
| FLP100359 | Human NK1R full length protein-synthetic nanodisc |
| FLP100370 | Human NPY1R full length protein-synthetic nanodisc |
| FLP100384 | Human OPRM full length protein-synthetic nanodisc |
| FLP100429 | Human OXYR full length protein-synthetic nanodisc |
| FLP100442 | Human F2RL2 full length protein-synthetic nanodisc |
| FLP100453 | Human QRFPR full length protein-synthetic nanodisc |
| FLP100157 | Human ADRB1 full length protein-synthetic nanodisc |
| FLP110098 | Human MBP-AGTR1 full length protein-synthetic nanodisc |
| FLP120031 | Human GPR75-Strep full length protein-synthetic nanodisc |
| FLP120175 | Human ACKR1-Strep full length protein-synthetic nanodisc |
| FLP120313 | Human GPR6-Strep full length protein-synthetic nanodisc |
| FLP120328 | Human HCAR2-Strep full length protein-synthetic nanodisc |
| FLP120360 | Human NK2R-Strep full length protein-synthetic nanodisc |
| FLP110031 | Human MBP-GPR75 full length protein-synthetic nanodisc |
| FLP100031A | Human β2AR-GPR75-BRIL full length protein-synthetic nanodisc |
| FLP120289 | Human GPRC5C-Strep full length protein-synthetic nanodisc |
| FLP100031C | Human BRIL-GPR75 full length protein-synthetic nanodisc |
If you are conducting membrane protein research, we invite you to explore our Nanodisc products to support your scientific endeavors! For more information, please contact 19789120878 !