Antibody-drug conjugates (ADCs) mainly consist of monoclonal antibodies, linkers, and cytotoxic payloads. The antibody, acting as the “missile” in ADC drugs, possesses target-specific functionality. It can selectively recognize tumor cell surface antigens, undergo internalization upon binding to these antigens, and deliver the cytotoxic payload to tumor cells to exert its toxic effects. Since not all antibodies internalize upon antigen binding, the internalization capability of antibodies is a crucial criterion in the early screening of ADC drugs. What are the principles of antibody internalization? What are the pathways of action? What factors influence antibody internalization? And what are the current methods for detecting antibody internalization?
1. Principles and Pathways of Antibody Internalization
As mentioned earlier, antibody internalization, or endocytosis, refers to the process by which antibodies on the cell surface bind to corresponding antigens and carry the antibody-antigen complex into the cell interior by their intrinsic molecular transport system. Antibody internalization is the primary mechanism for the entry of most ADC drug molecules into cells. Conventional endocytic processes can be divided into three stages: vesicle formation, membrane bending and vesicle maturation, and membrane fission and release into the cytoplasm. Analyzing the internalization pathways of currently approved ADCs (as shown in Figure 1), ADC internalization pathways can be classified into two categories based on whether they depend on clathrin-mediated endocytosis (CME): CME-dependent internalization and CME-independent internalization. The latter can be further divided into caveolae-mediated endocytosis, clathrin-independent carrier/GPI-anchored protein-enriched early endosomal compartment (CLIC/GEEC), and macropinocytosis.
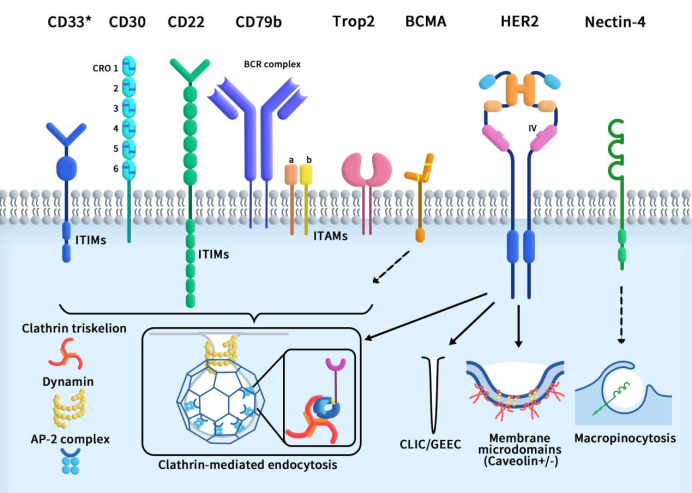
Figure 1 collection of endocytosis pathways utilized by the target antigens for the currently approved ADCs [1]
Here, we will focus on discussing the primary pathway of ADC drug internalization—Clathrin-mediated endocytosis (CME). CME comprises a series of continuous and partially overlapping steps, with the initiation of CME varying among different receptors. This process can be triggered by the formation of specific receptors on the plasma membrane or by the binding of receptors to ligands and/or antibodies. The onset of CME occurs as Clathrin coat proteins aggregate on the plasma membrane. These coat proteins further assemble and grow by recruiting additional adaptor proteins from the cytoplasm. Crucial adaptor proteins induce membrane bending, leading to the accumulation of internalized receptors/ligands in Clathrin-coated pits (CCP). As CCP invaginates, the CCP neck contracts, followed by a rupture process that separates it from the plasma membrane. Actin polymerization facilitates the inward movement of CCP into the cytoplasm until the division is complete, releasing CCP as clathrin-coated vesicles (CCV). Ultimately, the CCV shell undergoes degradation, CCV fuses with endosomes, and it is directed to specific subcellular locations or recycled back to the cell surface [1].
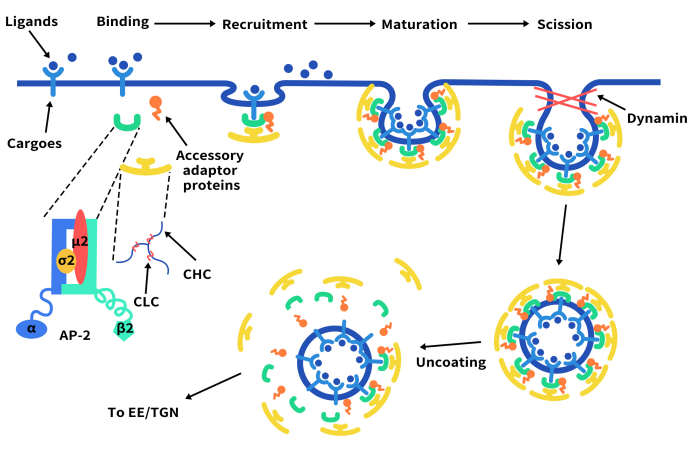
Figure 2. The mechanism of Clathrin-mediated endocytosis
2. What are Factors Influencing Antibody Internalization
Whether an antibody can be internalized is mainly determined by the target, and the efficiency of antibody internalization is influenced by various factors.
Antibody affinity and specificity: Antibodies with high affinity can bind to antigens more effectively and promote internalization. Antibodies with high specificity can recognize antigens more accurately and avoid non-specific binding. However, it is noticeable that antibodies with extremely high antigen affinity will reduce the penetration efficiency of solid tumors and decrease the efficiency of ADC drugs reaching the interior of the tumor.
Types and subtypes of antibodies: Different types and subtypes of antibodies have different receptors and signaling pathways, which make difference in the speed and efficiency of internalization. For example, IgG and IgA are internalized faster, while IgM and IgE are internalized slower.
Antibody dose and concentration: The higher the antibody dose and concentration, the more it will bind to the antigen and the greater the likelihood of internalization. However, too high an antibody dose and concentration may also lead to receptor saturation or downregulation, reducing the effectiveness of internalization.
Cell type and status: Different types of cells have varied receptor expression and internalization abilities, which affect the differences in antibody internalization. For example, B cells and macrophages have strong internalization capabilities, while T cells and red blood cells have weak internalization capabilities. The state of the cell also affects the kinetics of internalization, for example, activated cells internalize faster, while apoptotic cells internalize slower.
Additionally, larger antigen molecular weights are generally more challenging to internalize. Even different antibodies targeting the same antigen may exhibit different internalization efficiencies. Therefore, in the development of ADC drugs, selecting antibodies with high internalization efficiency is crucial for safety.
3. Methods for Detecting Antibody Internalization
Various conventional methods are used to detect antibody internalization, categorized into four types based on experimental types: live-cell imaging-based internalization detection, toxin-conjugated killing detection, pH probe-based internalization detection, and temperature-shift-based fluorescence secondary antibody internalization detection.
Live-cell imaging-based internalization detection, also known as the Incucyte method, involves real-time monitoring of naked antibodies for the real-time detection of pharmacokinetics. It is the preferred choice for antibody internalization detection in a multi-concentration and multi-timepoint mode, allowing real-time observation of live cells for up to 72 hours.
Toxin-conjugated killing detection mainly includes two methods: the DT3C method and the Mab-ZAP method. Both methods involve inducing cell toxicity by releasing toxins inside the cells through antibody-toxin complexes. DT3C, a recombinant protein produced through genetic recombination, consists of a diphtheria toxin (DT) without a receptor-binding domain and the C1, C2, and C3 (3C) domains of streptococcal protein G. Mab-ZAP is composed of mouse antibodies and ribosome-inactivating protein saporin. Compared to the traditional Mab-ZAP method, DT3C has a more stable molecular weight, higher internalization efficiency, broader applicability, and lower cost.
Internalization detection based on pH probes and fluorescent secondary antibody internalization detection based on temperature transition are two commonly used methods to detect the internalization process of cells. They both have their own advantages and disadvantages. Internalization detection based on pH probes uses the sensitivity of fluorescent probes to pH value to reflect the degree of acidification of intracellular vesicles, thereby determining the process and location of internalization. Fluorescent secondary antibody internalization detection based on temperature transition uses the fluorescence intensity changes of fluorescent secondary antibodies at different temperatures to distinguish the fluorescence signals inside and outside the cells to determine the efficiency and extent of internalization. Both methods have the following advantages and disadvantages:
| Internalization detection based on pH probes | Fluorescent secondary antibody internalization detection based on temperature transition | |
| Advantages | Simple and easy to operate without complex instruments and reagents. The fluorescence signal is clear and can be analyzed quantitatively, making it suitable for high-throughput screening. Can be used with a variety of cell types and a variety of fluorescent markers, such as antibodies, ligands, drugs, etc. | |
| Disadvantages | Appropriate pH-sensitive probes need to be selected to match different internalization pathways and targets; Different pH probes have different pH response ranges and sensitivities, as well as different fluorescence properties and stability; The pH probe may be interfered by other factors, such as other fluorescent substances in the cell, buffer systems inside and outside the cell, etc. | It is necessary to control temperature changes to ensure the reliability of fluorescence signals; Different fluorescent secondary antibodies have different temperature sensitivities, as well as different fluorescence properties and stability; Temperature shifts may affect the physiological state of cells and the kinetics of internalization, as well as the binding and release of fluorescent markers. |
4. DiTag pH-Sensitive Reagent Facilitating Antibody Internalization Detection
The pH-sensitive IgG labeling reagent developed by DiTag provides a convenient solution for testing antibody internalization. The two reagents (AME100001 and AME100002) utilize pH-sensitive fluorescence-labeled Fc-binding protein, which forms a complex with IgG antibodies from different species. After antibody internalization, the surrounding pH becomes acidic, enhancing the fluorescence signal of the antibody-reagent complex. The intensity of the fluorescence signal directly reflects the efficiency of antibody internalization. By measuring the fluorescence signal strength, researchers can assess the efficiency of antibody internalization. The primary distinction between AME100001 and AME100002 lies in the specific IgG types they are designed to label. To summarize:
| AME100001 | AME100002 | |
| Specific IgG types | Human IgG1, IgG2, and IgG4; Rabbit IgG; Mouse IgG2a and IgG2b. | Human IgG1, IgG2, IgG3, and IgG4; Rabbit IgG; Mouse IgG1, IgG2a, IgG2b, and IgG3. |
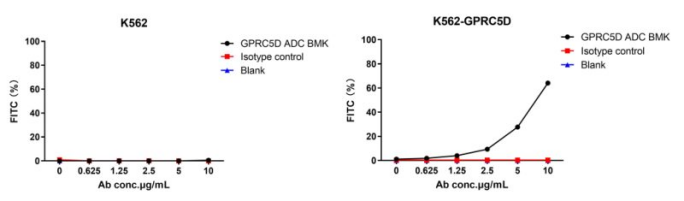
Figure 3. The fluorescent signal from GPRC5D ADC BMK-AME100001 conjugate is only detected in GPRC5D positive cells (K562-GPRC5D stable expression cell line), indicating specific internalization.
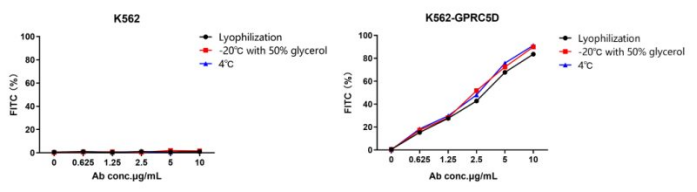
Figure 4. Stability test of AME100001. Three storage methods are tested: lyophilization and reconstitution (black), liquid with 50% glycerol at -20℃ (red), liquid at 4℃ (blue). All three methods exhibit excellent stability.
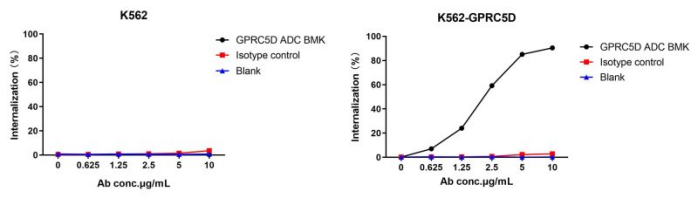
Figure 4. The fluorescent signal from GPRC5D ADC BMK-AME100002 conjugate is only detected in GPRC5D positive cells (K562-GPRC5D stable expression cell line), indicating specific internalization.
Reference:
[1] Hammood M, Craig AW, Leyton JV. Impact of Endocytosis Mechanisms for the Receptors Targeted by the Currently Approved Antibody-Drug Conjugates (ADCs)-A Necessity for Future ADC Research and Development. Pharmaceuticals (Basel). 2021 Jul 15;14(7):674.
