Dual-Function Reagent for ADC Screening: Evaluating Internalization and Cytotoxicity in One Step
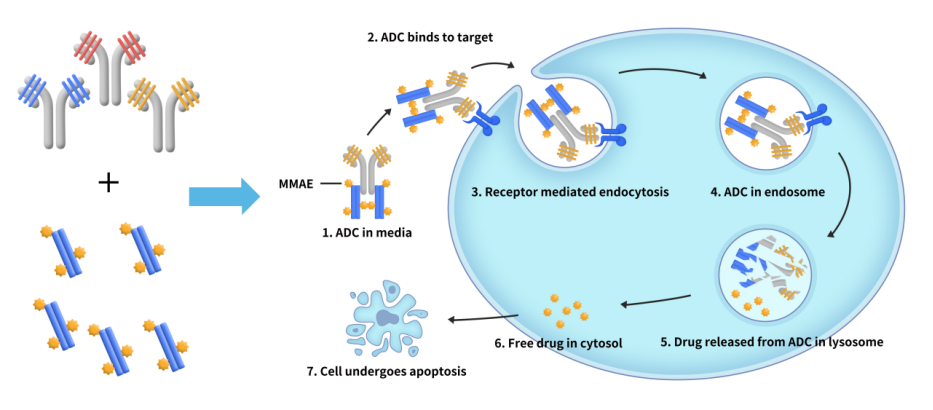
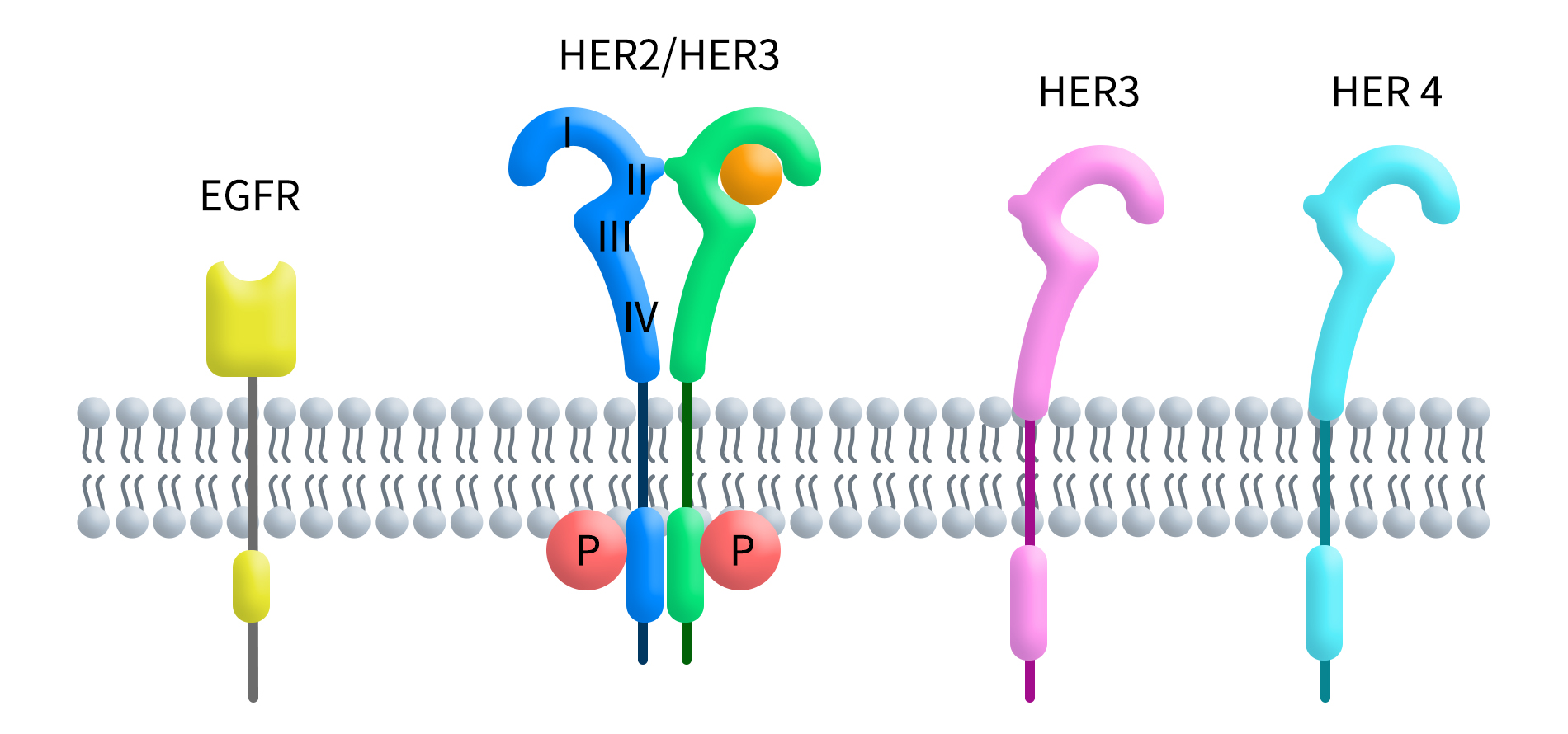
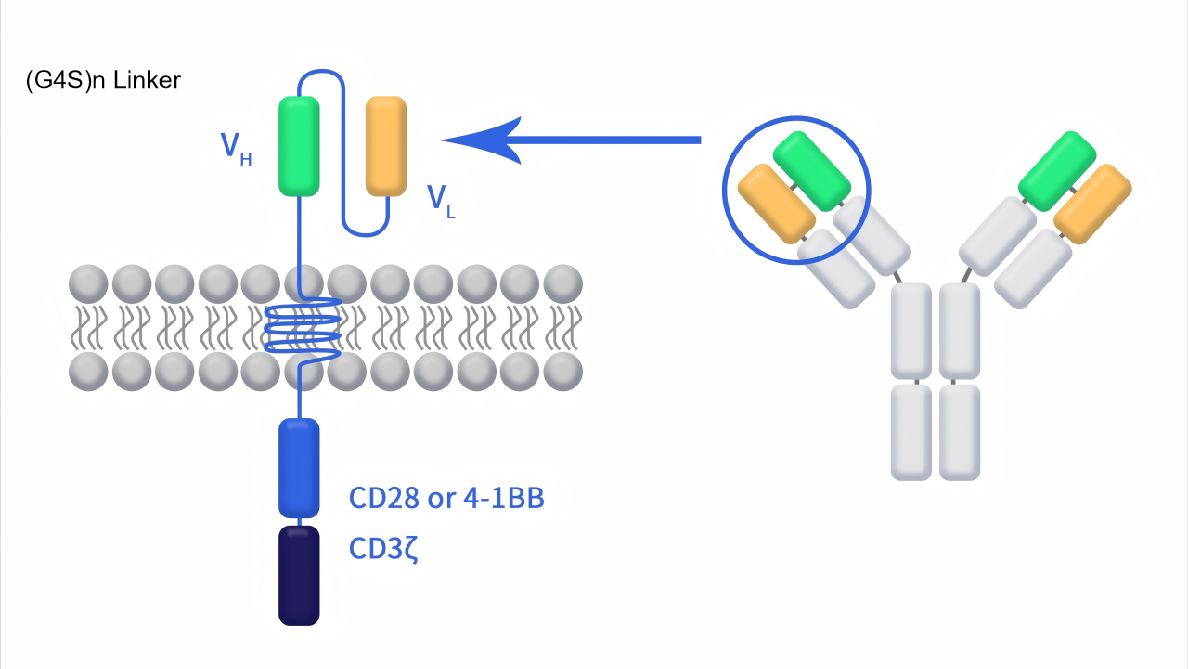
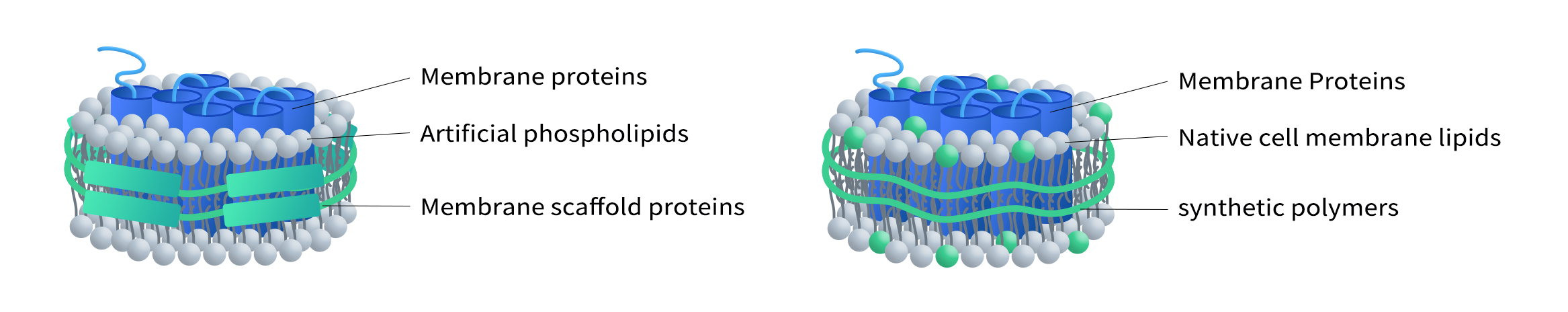
In recent years, Antibody-Drug Conjugates (ADCs) have gained widespread attention as a key strategy in targeted cancer therapies. The effectiveness of ADCs relies on the specific targeting of the antibody, efficient internalization, and the release of the payload toxin. Therefore, evaluating both the internalization efficiency and cytotoxicity introduced by antibody-payload complex is crucial in ADC […]