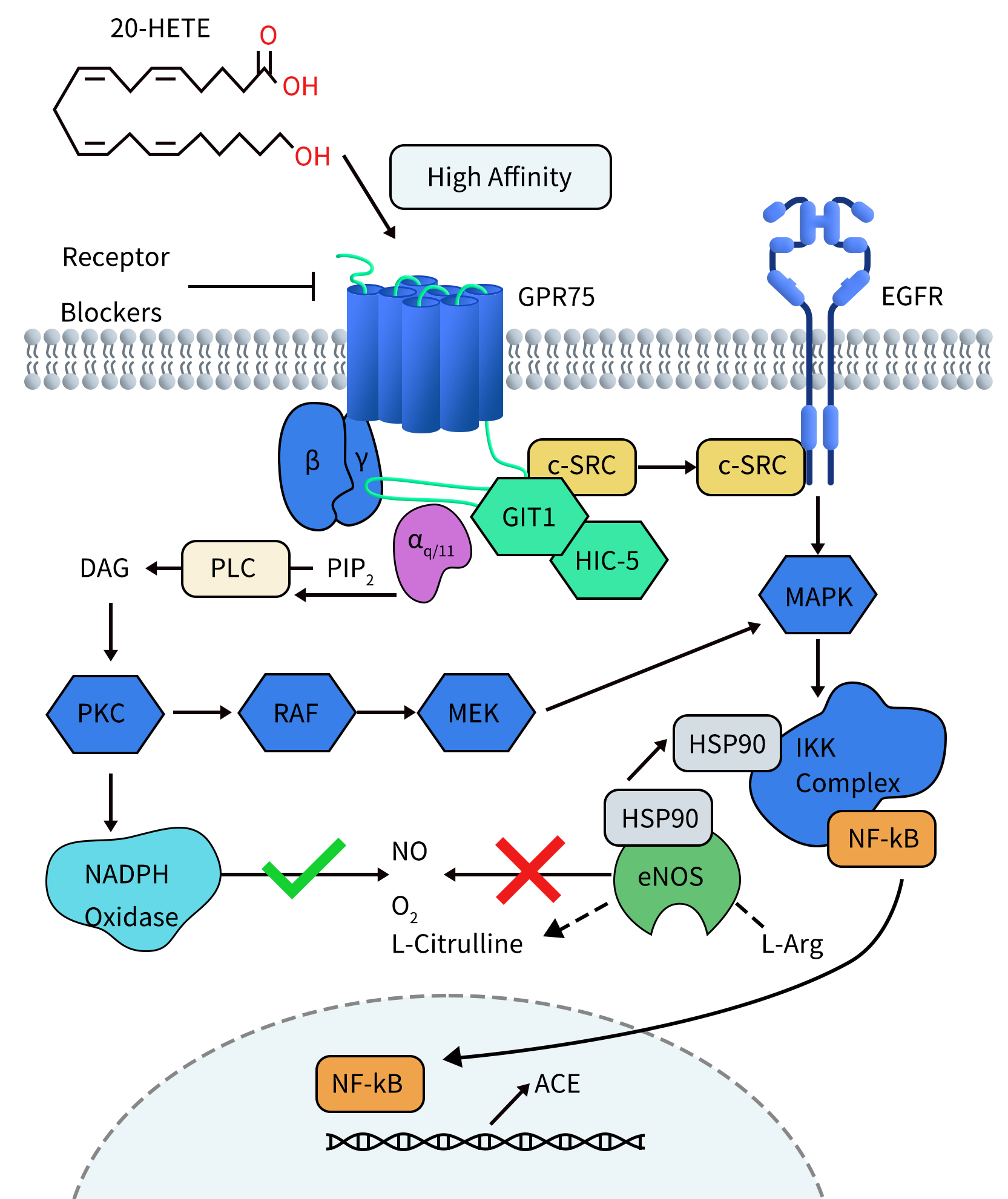
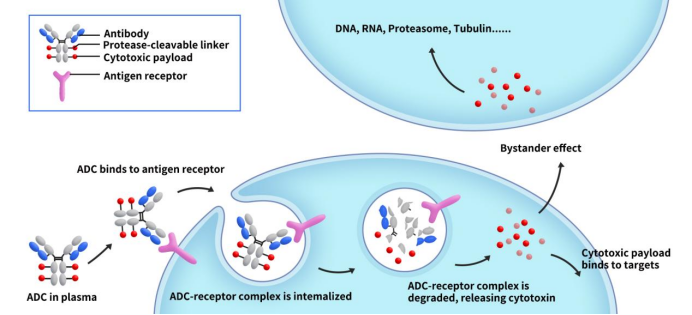
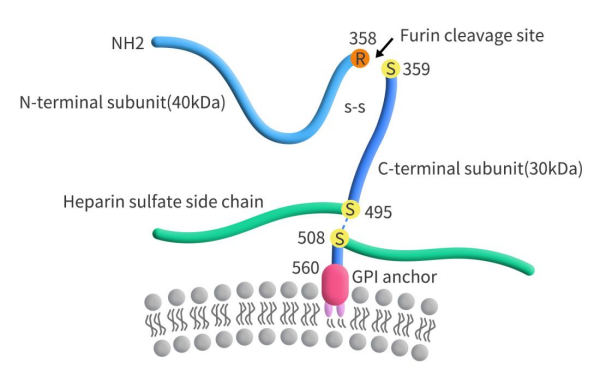
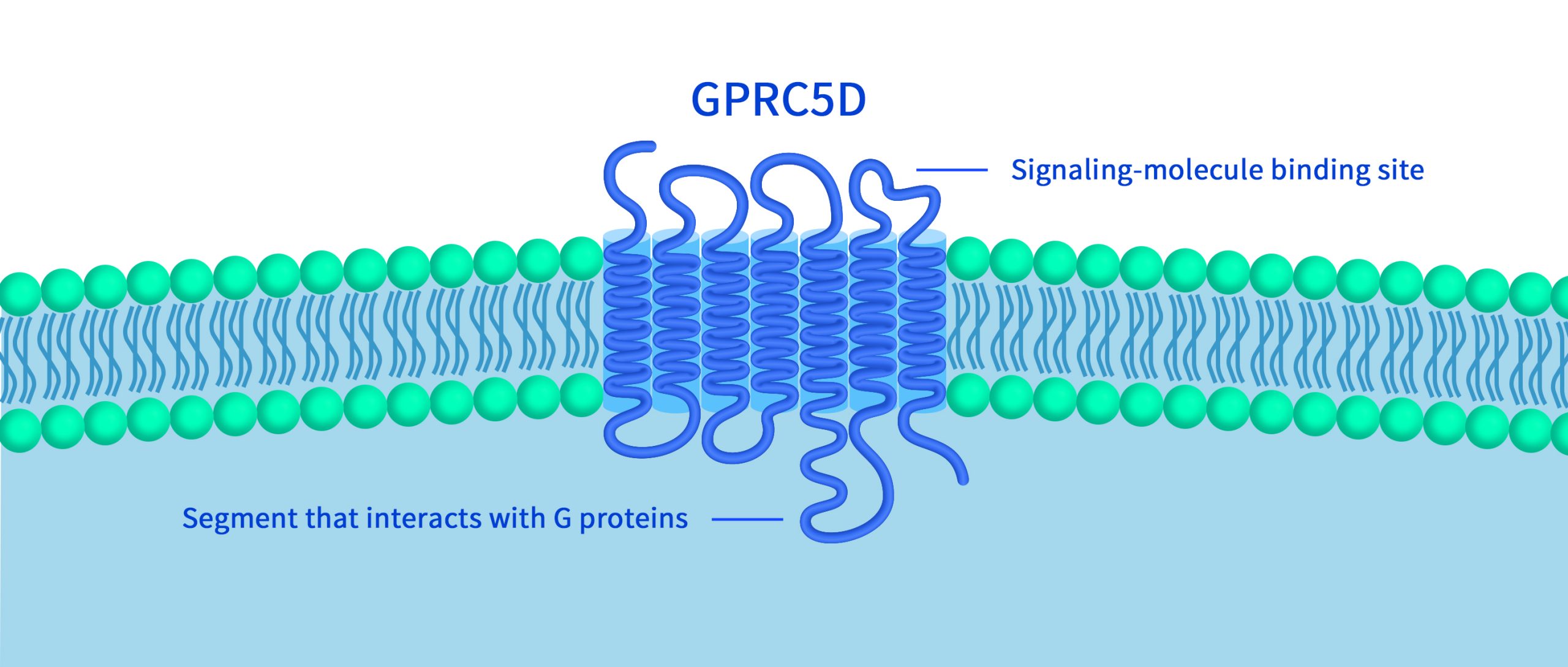
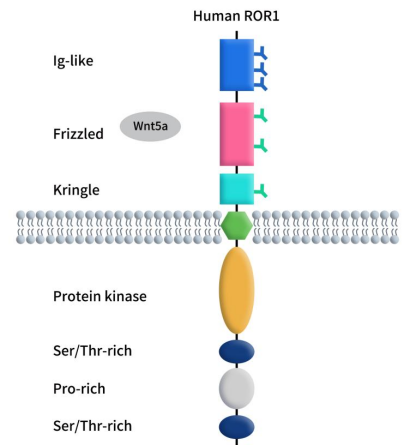
GPR75 – An Emerging Target for Weight Loss Drugs
When it comes to weight loss medications, one cannot overlook the remarkable success of Semaglutide (GLP-1 weight loss drug), which has propelled the Danish biopharmaceutical company, Novo Nordisk, to new heights. According to Novo Nordisk’s financial report for 2023, Semaglutide sales reached $21.2 billion in 2023. On March 7th, Novo Nordisk’s stock price surged by […]