Applications
01
Immunogens for antibody drug development
02
Reagents used for CAR T positive cell monitoring
03
Reagents for antibody screening and functional testing
04
Reagents for antibody affinity measurement
Immune checkpoints are a class of proteins that regulate tumor immune microenvironment. They can be classified into two categories, costimulatory immune checkpoints, such as OX40, GITR, ICOS, etc.; and co-suppressive immune checkpoints, such as PD1, CTLA-4, etc.. Right now there are a number of FDA approved drugs on co-suppressive targets, such as Yervoy, anti-CTLA-4 monoclonal antibody, Keytruda, anti-PD-1 monoclonal antibody and Tecentriq, anti-PD-L1 monoclonal antibody.
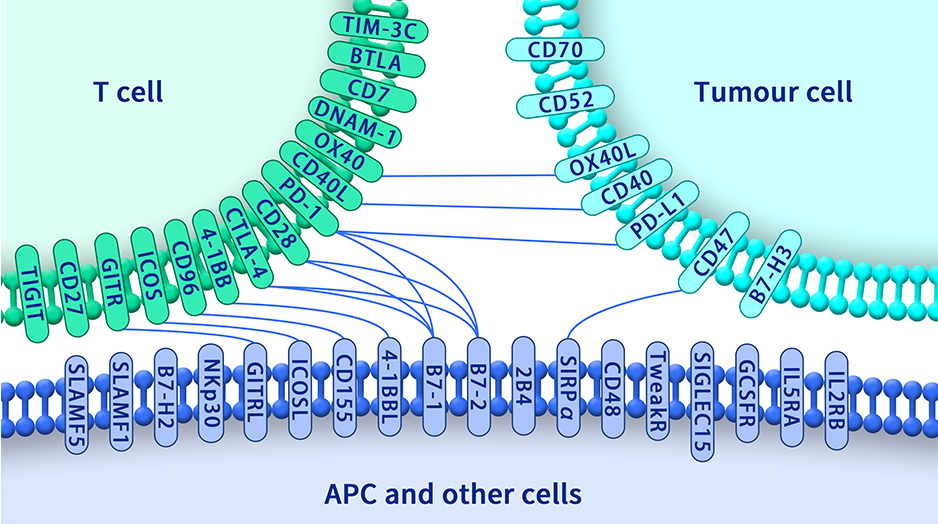
Therapeutic antibodies need to exert their functional activities in human body. Therefore, it is very critical to identify monoclonal antibodies with functional activities. “Garbage in Garbage out” is the rule of thumb for therapeutic antibody development. DIMA Biotechnology LTD pays special attention on its immunogen development process. All the proteins were made by using HEK293 mammalian cell secretion expression system and we implemented a strict quality control process, including purity testing, antibody-drug interaction verification, freezing and thawing Tests, thermal stability tests, etc. Biopharms are actively looking to identify new co-suppressive or co-stimulatory targets for therapeutic drug development.
Immunogens for antibody drug development
Reagents used for CAR T positive cell monitoring
Reagents for antibody screening and functional testing
Reagents for antibody affinity measurement
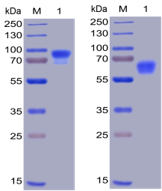
Figure 1. Human SIRPa, hFc-His Tag (left) and CD47, mFc-His Tag (right) on SDS-PAGE under reducing condition.
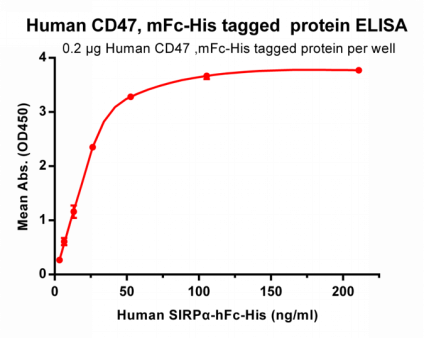
Figure 2. ELISA plate pre-coated by 2 ug/ml (100 ul/well) Human CD47, mFc-His tagged protein (PME100008) can bind its native ligand Human SIRPα, hFc-His tagged protein (PME100009) in a linear range of 3.3-26.37 ng/ml.
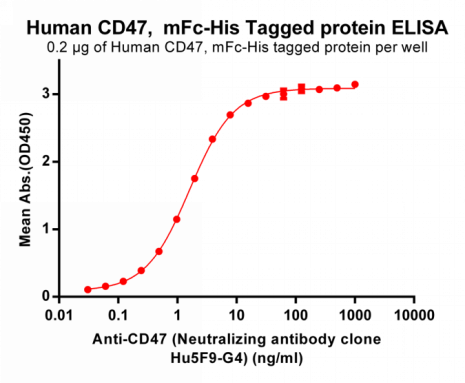
Figure 3. ELISA plate pre-coated by 2 ug/ml (100 ul/well) Human CD47, mFc-His tagged protein (PME100008) can bind Magrolimab (Neutralizing antibody clone Hu5F9-G4) in a linear range of 0.061-1.606 ng/ml.