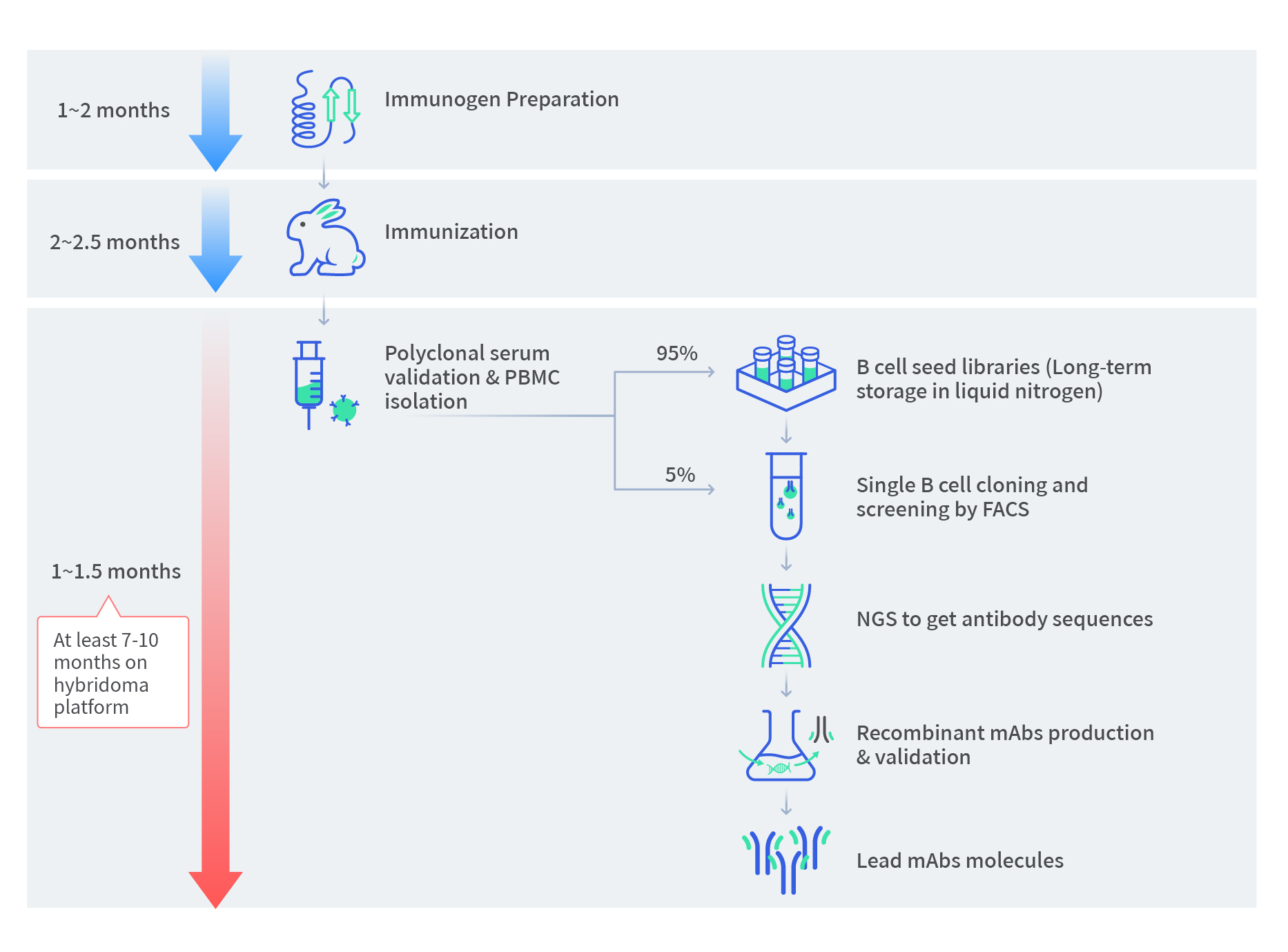
Background of Therapeutic Lead Antibody Molecules
Monoclonal antibodies (mAbs) can be used as “magic bullets” for the treatments of cancer and other diseases due to their specificities. They are the fastest growing class of drugs over the past decade. The process of antibody-based drug discovery is lengthy and costly, requiring many years of development and millions or even billions of dollars of investment (Figure 1).
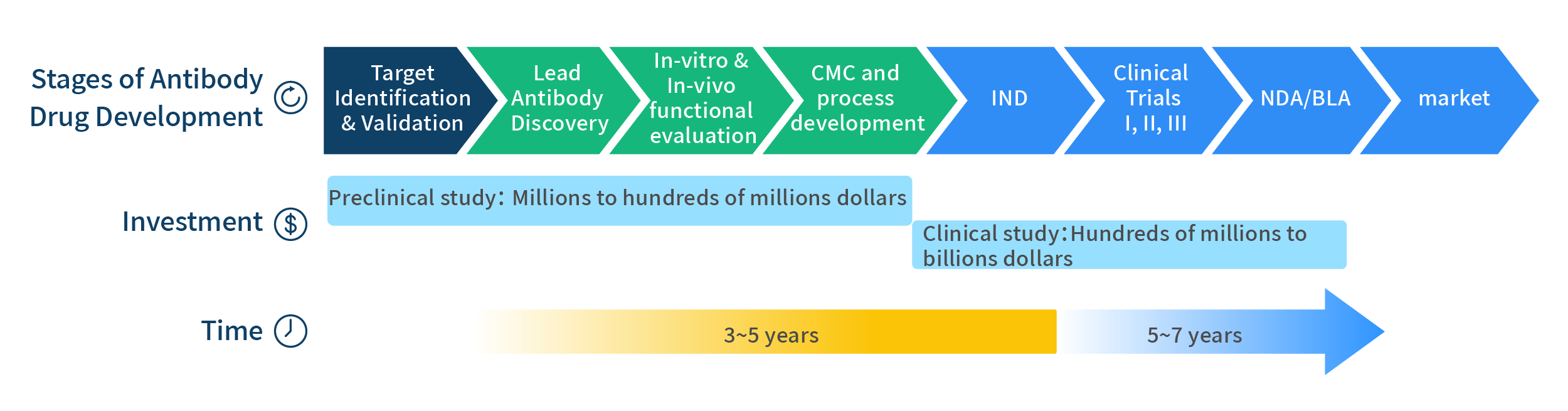
Figure 1: This schematic shows investment and time required for the preclinical and clinical stages of antibody drug development. The process of antibody drug development is very costly and lengthy. A good lead mAb molecule can be the entry key to the process.
Challenges For Lead Antibody Molecule Selection
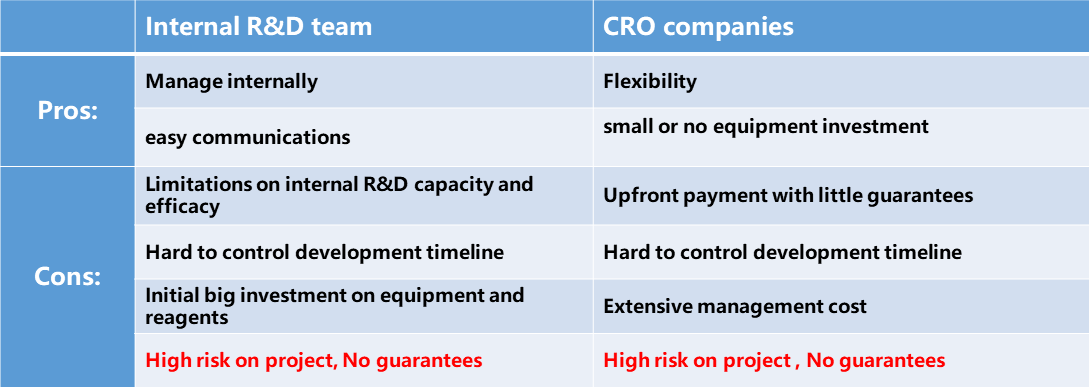
With recent scientific discovery on new mechanism of immune-oncology, more and more potential drug development candidates are on the horizon. How to quickly finish pre-clinical development and push to clinical stage becomes Biopharma’s top priority. The valuation of the company is highly dependent on whoever makes the first positive clinical report. In the meantime, more innovative antibody drug formats, such as bi-specific antibody, ADC, etc., were explored and tested in clinical trials. To screen for new lead antibody molecules to adapt into new therapeutic antibody format is in urgent need. All these will push Biopharma to drive fiercely on its pre-clinical development capacity. To Scale up internal R&D team or contract-out to CROs become the new normal for Biopharma. Both options have their Pros and Cons, as summarized in the table 1. Due to the complexity and unpredictability of therapeutic lead mAb discovery process, there will be no guarantees of success at this stage, which impose a bottleneck for the current antibody drug development (Table 1).
Table 1. Current choices for Biopharma to develop lead mAb molecule