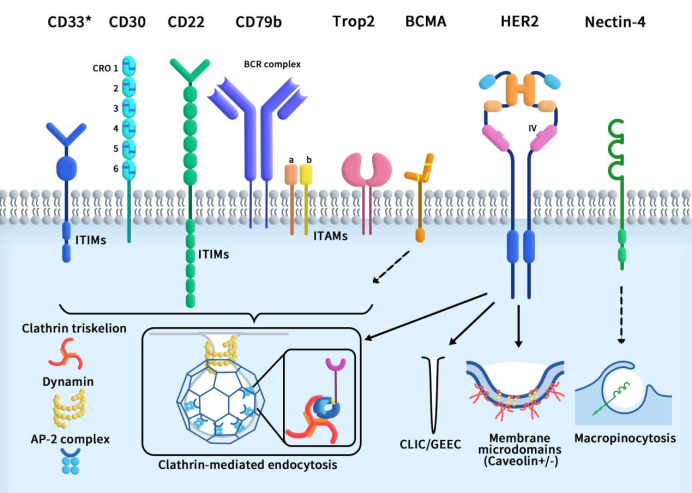
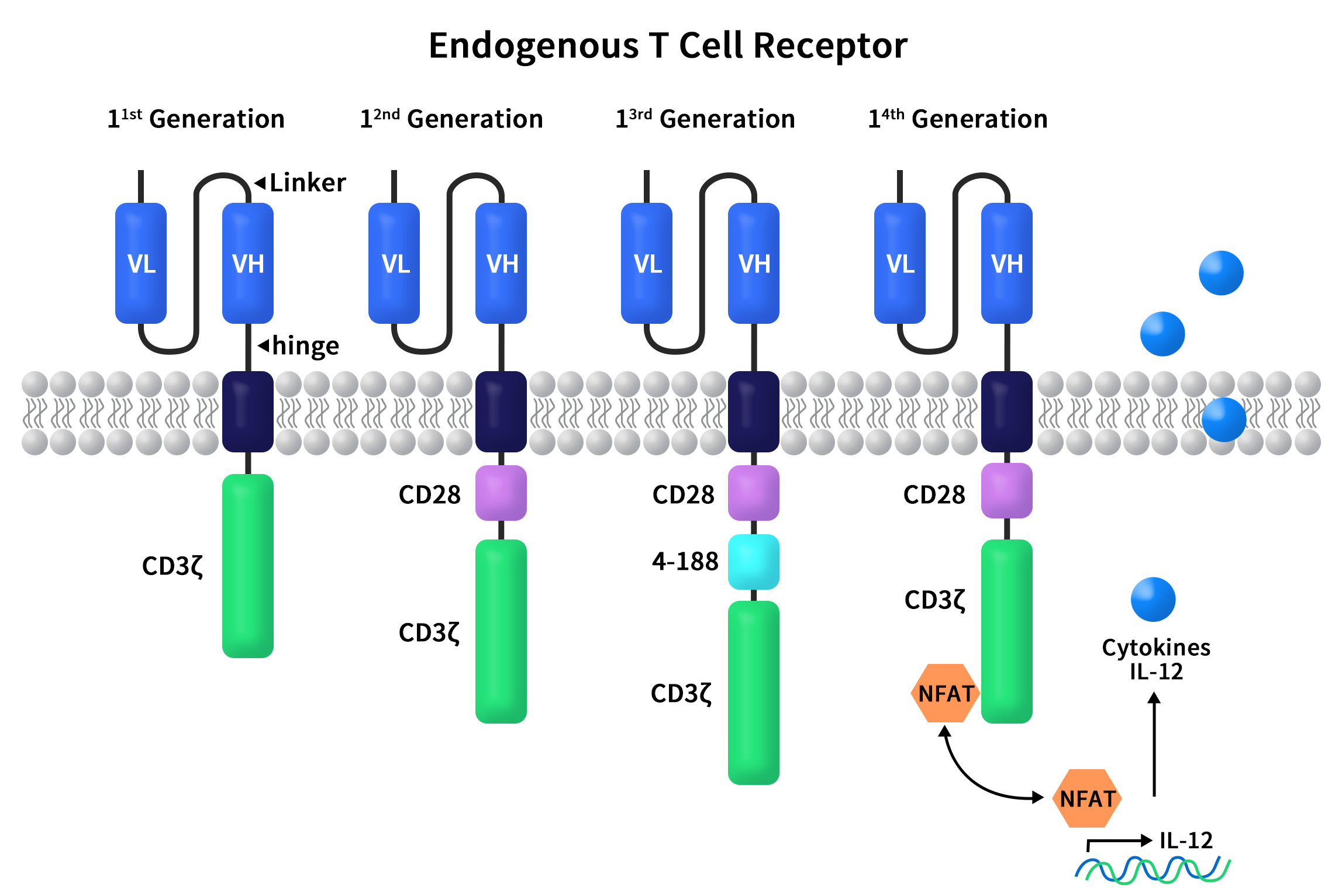
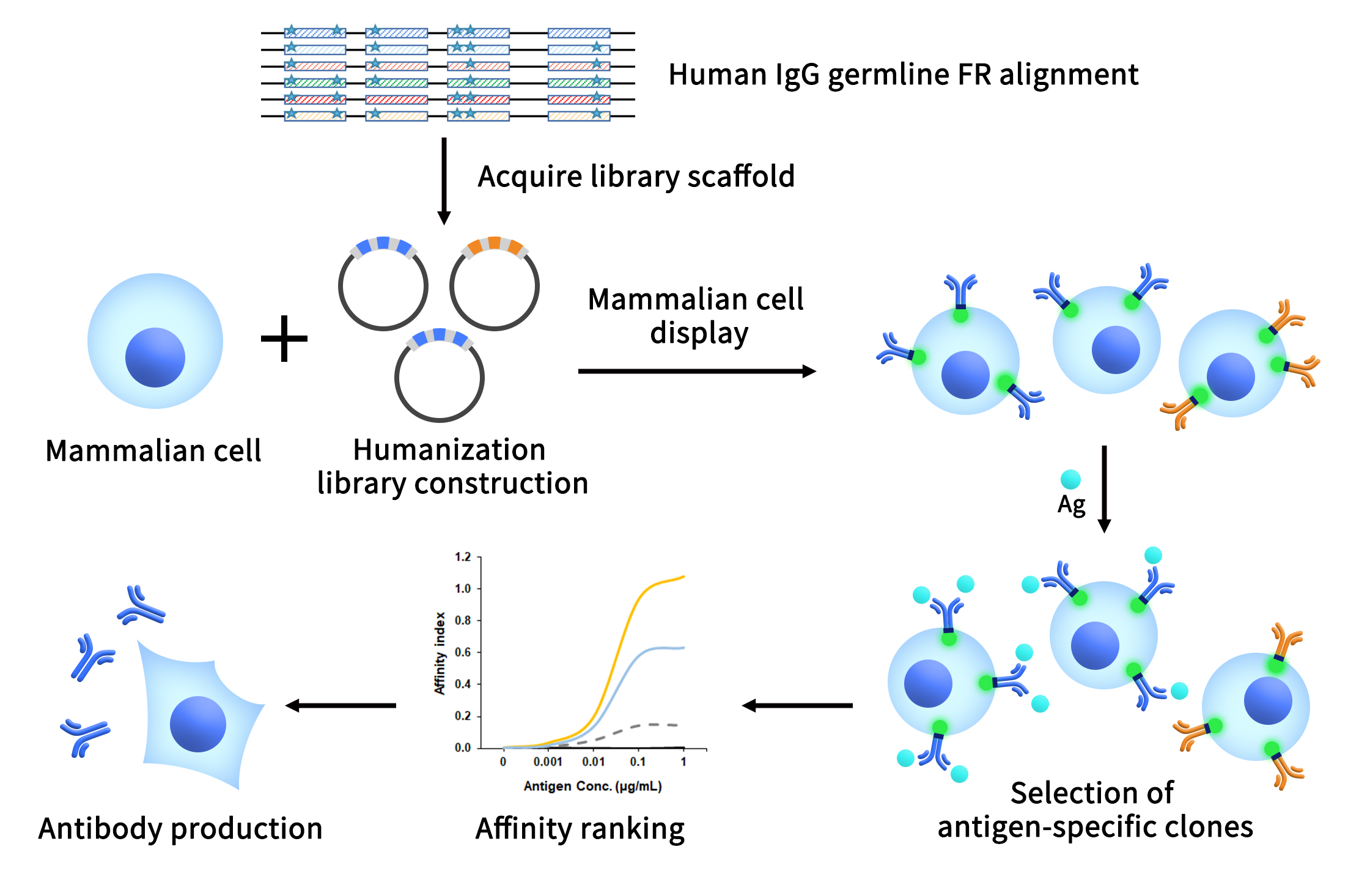
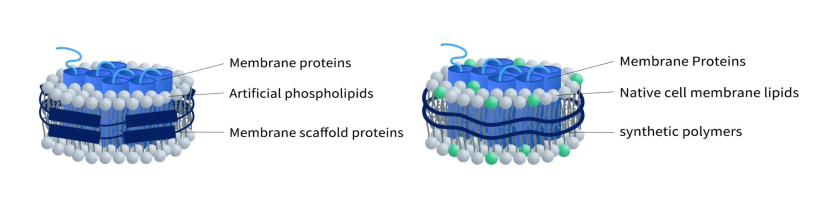
Understanding Single B Cell Cloning Technology in One Read
Antibody drugs, due to their high specificity and significant therapeutic effects, possess superior pharmacokinetic properties, making them a crucial player in the biopharmaceutical market. According to Frost & Sullivan’s statistical predictions, the global antibody drug market is expected to reach $443.1 billion by 2030. As the demand for antibody drugs continues to grow, the development […]