CD28
CD28, as the first discovered costimulatory receptor, is the founding member of a subfamily of costimulatory molecules characterized by extracellular variant immunoglobulin-like domains. CD28 mainly acts as a “second signal” (binding to B7-1/ B7-2 on the surface of target cells) to lower the threshold required for effective activation of T cells, and to enhance the “first signal” of T cell activation (TCR-CD3 complex recognition and binding of MHC-polypeptides on target cells). It promotes the T cells to further develop and proliferate into cells with immune functions.
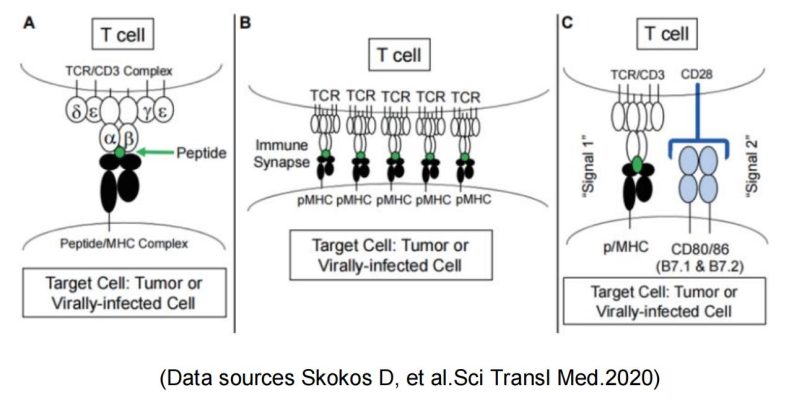
Meanwhile, CD28 and the coinhibitory receptor CTLA-4 with their shared ligands B7-1 and B7-2 constitute the most clearly characterized regulatory T cell pathway and are examples of other costimulatory and coinhibitory pathways.
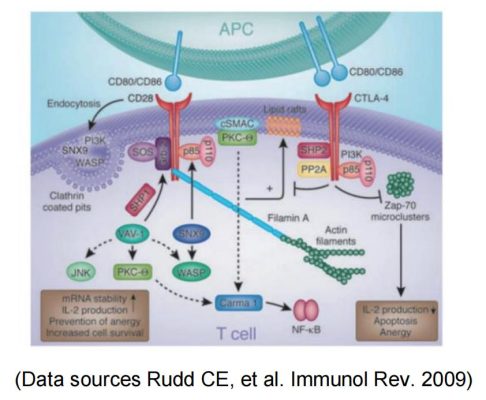
Based on the importance of CD28 costimulation for T cell activation, immunoregulation by activating or blocking the CD28/B7-1 (CD80)/B7-2 (CD86) pathway is a promising approach: Blockade of costimulation can effectively prevent T cell activation and reduce the risk of allograft rejection during transplantation. It can also be used for the treatment of T cell-mediated autoimmune diseases.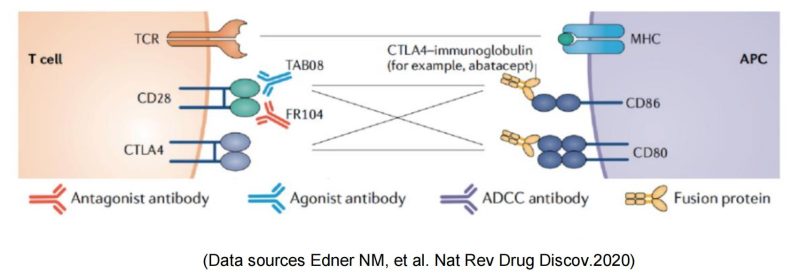
CD28 target development
The molecular mechanism of CD28 is clear, and as an old target, both antagonists and agonists can be developed for different clinical indications, which make CD28 a promising drug target. However, currently there are only a few antibody drug development pipelines, mainly related to the early clinical incidents of the CD28 agonist TGN1412. In a phase I clinical trial of the CD28 superagonist antibody TGN1412 (developed by TeGenero and bankrupt after trial failure) for the treatment of rheumatoid arthritis at March 2006, six volunteers suffered life-threatening cytokine release syndrome that was completely not predicted in the preclinical animal model studies. The main reason from the subsequent analysis indicated the experimental animals were too “clean” and did not accumulate enough effector T cells (TEM cells, the main source of pro-inflammatory factors). In addition, the lack of CD28 on the macaque effector T cell caused significant deviations in the dosing guidelines for the clinical stage. This serious clinical incident directly affected the expectation of CD28.
CD28 Research and Development pipeline
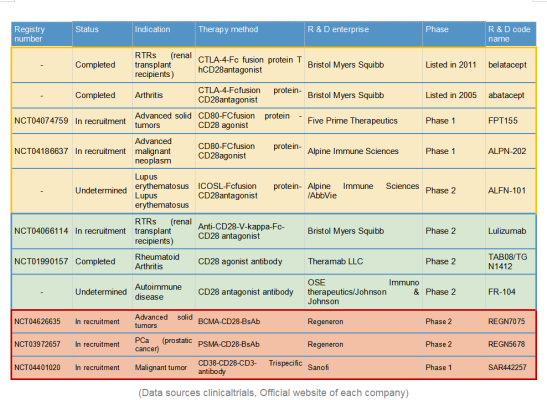
SAR443216
Current clinical drug development targeting CD28 is focused on 3 areas: CD28 associated target based recombinant protein drugs; Monoclonal antibody drugs (MAbs); Multi-specific antibody drugs. Below let’s take a look one by one.
- CD28 associated target based recombinant protein drugs:
Various fusion -FC recombinant proteins have been developed based on CD28-associated proteins such as B7-1 (CD80)/B7-2 (CD86)/B7-H2 (ICOSL), CTLA4 et al, either as native ligand and co-ligand for competition.
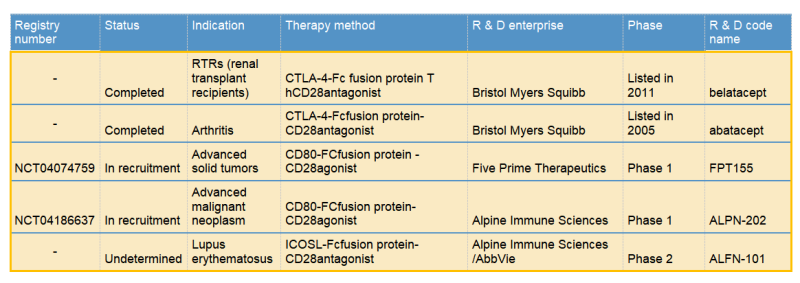
Bristol Myers Squibb & abatacept/belatacept
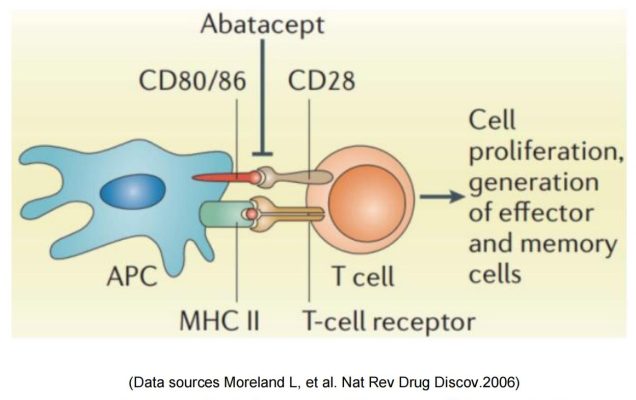
Two commercialized CTLA-4-Fc fusion proteins developed by Bristol Myers Squibb, can inhibit the activation of T cells by binding to the costimulatory molecules CD80 and CD86 on the antigen presenting cells (APC) and blocking the interaction between CD80/CD86 and CD28.
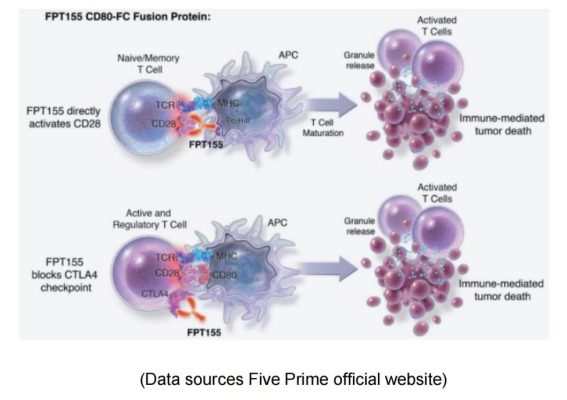
Five Prime & FPT155
Five Prime is a clinical stage biotechnology company focused on the research and development of protein therapies. Its FPT155 uses soluble CD80 fusion protein: directly participates in CD28 costimulatory activity; It also prevents CTLA-4 from competing with endogenous CD80, thereby allowing CD28 signaling to dominate T cell activation.
Alpine Immune & ALPN-101/ALPN-202
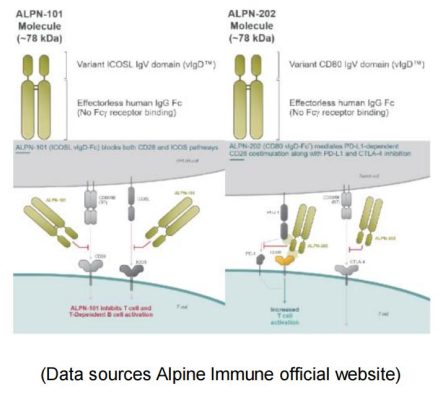
Alpine Immune is a leading clinical-stage immunotherapy company focused on the discovery and development of protein-based immunotherapies. Among their multiple pipelines, ALPN-101 and ALPN-202 are very unique, which represent the perfect examples for the development of CD28 agonist/ antagonist fusion proteins.ALPN-101, a dual inhibitor of CD28 and ICOS-T costimulatory pathways, improves the prognosis of patients with severe autoimmune/ inflammatory diseases by simultaneously blocking two key costimulatory pathways. ALPN-202 binds to PD-L1 on tumor cells and prevents PD-L1 / PD-1 interaction. Meanwhile it also drives T cell activation by binding to CD28 (PD-L1-dependent CD28 costimulation). ALPN-202 also binds to CTLA-4 on T cells and blocks CTLA4-CD80 / CD86 interactions, thereby further enhancing T cell activity. ALPN-101 has attracted AbbVie to sign an $865 million global exclusive option and license agreement with Alpine Immune.
- Monoclonal antibody drugs(MAbs)
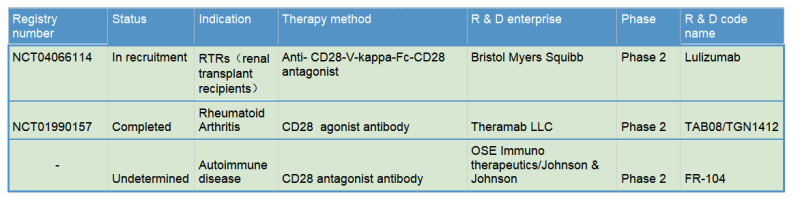
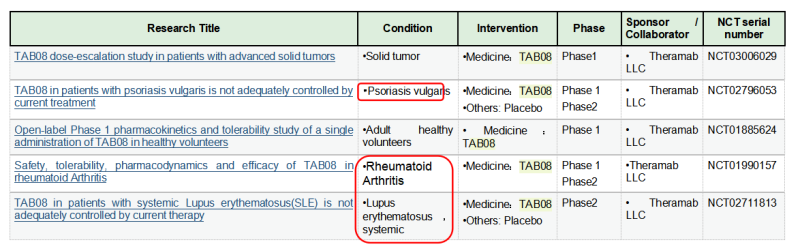
TheraMab & TGN1412 Reborn
The TGN1412 incident also indicates the potent capability of T cell activation from CD28 agonist antibodies. Therefore, TGN1412 remained active after later it was acquired by TheraMab, a Moscow-based biological company, and was renamed as TAB08. At the same time, based on the original dose, clinical trial was continued at a reduction to 1/1000 dose of TG1412: Phase 1 safety trials have shown well tolerance, and multiple phase 2 trials have been completed, but the results are late to be published. Moreover, TAB08’s indications are mostly autoimmune/inflammatory diseases, which, one might be surprised to learn, is in apparent conflict with the function of other CD28 agonists since other agonists mainly play a role in immune enhancing and proinflammatory effects. This may be mainly because TAB08 is cautiously down-regulated at low doses, selectively target activation of regulatory T cells (T-Reg) preferentially immunosuppressive T cell subsets over traditional T cells. However, the specific mechanism is still not clear: early studies have shown (Zheng Y, et al. J Immunol. 2004) that CD28 activation signals on the surface of regulatory T cells (T-Reg) inhibit the suppressive function of Treg. If TAB08 acts as a selective target to activate CD28+Treg, it should down-regulate the suppressive function of Treg, which also loses its significance in the treatment of autoimmune/inflammatory diseases. It also conflicts with the mechanism of action of FR104 below.
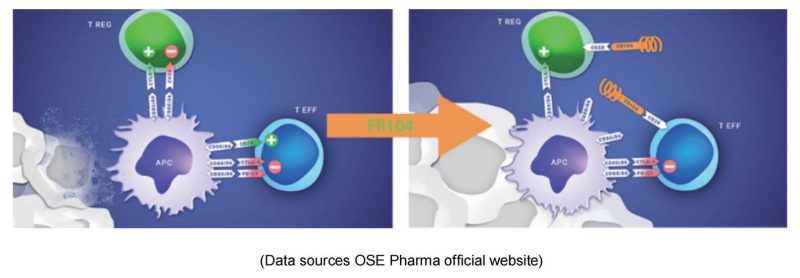
OSE Immuno & FR-104
OSE Pharma is a Paris-based biological company that develops specific immunotherapies to activate T lymphocytes. As a monoclonal antibody antagonist for CD28, FR104 binds to the overlapping epitope MYPPPY of CD28 ligand-binding motif and inhibits the binding pathway of CD28 to B7-1/ B7-2, therefore, down-regulates effector T cells, and promotes the activity of regulatory T cells (T-Reg). Phase 1 clinical studies have shown efficacy, a favorable safety profile and preliminary signals of Phase 2 recommended dosing, further supporting continued clinical development of this asset in autoimmune diseases or transplantation. Johnson & Johnson received the FR-104 global R & D and commercialization rights license at $173 million.
- Multispecific antibody drugs:
CD28 multispecific antibody drugs are the latest research and development results of Sanofi and Regeneron in the past two years. This design can reduce the cytokine release syndrome (CRS) caused by the dosage problem of CD28 monotargeted activating antibody, and maximize the activation of T cells with a low dose; Meanwhile this new approach can bring T cells and target cells together and make antitumor more efficient. Both companies have given the CD28 antibody a new glow.
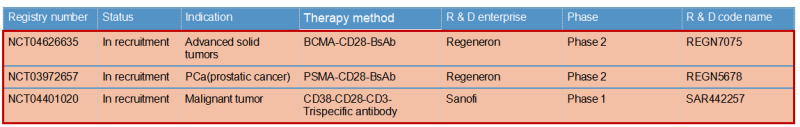
Regeneron & REGN7075 / REGN5678
Regeneron published the preclinical research and development data of REGN5678 (PSMA-CD28 bispecific antibody) in 2020: costimulatory CD28 dual antibody, paired with CD3 bispecific antibody in animal experiments obviously enhanced the efficacy with no risk of cytokine release syndrome. Based on this, the REGN7075 (BCMA-CD28 bisspecific antibody) pipeline was expanded and is now progressing steadily and rapidly to Phase 2.
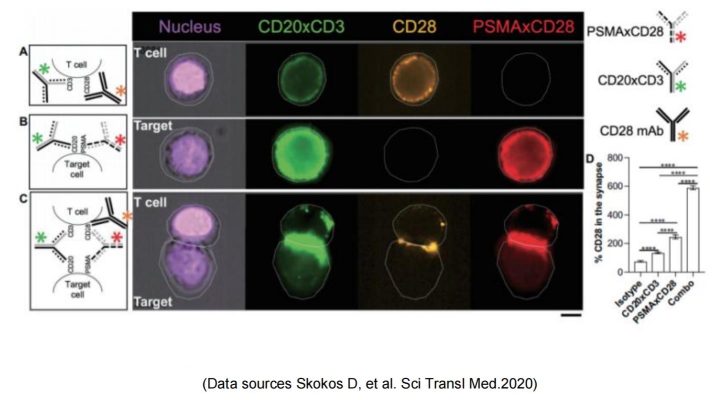
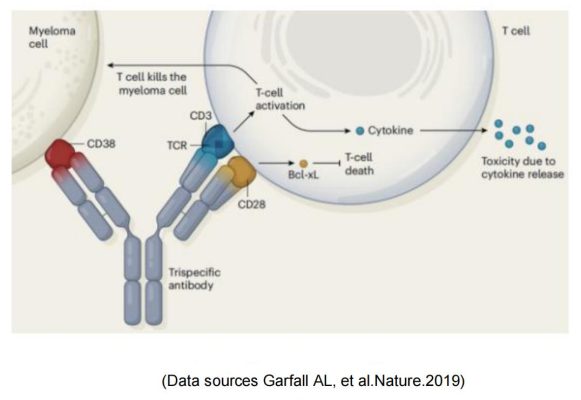
Sanofi & SAR442257
Sanofi introduced CD28 on the basis of traditional CD3 bispecific antibody to form a trispecific antibody SAR442257 that simultaneously targets CD38/CD3/CD28. SAR442257 significantly improved the ability of T cells to target multiple myeloma cells, and then activated T cells through the “first signal” pathway of TCR/CD3 and the “second signal” pathway of CD28, thereby exhibiting strong antitumor activity.
B7-H3 brief summary
It’s critical to understand how CD80 and CD86 ligands interact with CD28 and CTLA-4 for immune activation or inhibition in the development of CD28-targeting modulators; Regeneron and Sanofi have set a good example for using CD28’s potent T cell activation capability while reducing the risk of cytokine storm caused by the activation of T cells. Further data from different pipelines will make CD28 a hot drug target again.
At present, Dima Biotech have successfully completed the preparation of CD28 functional antigen, as well as the CD28-DimAb B cell seed bank, which can rapidly screen CD28 agonist or inhibitor antibody molecules, greatly saving your research and development cycle. Please contact us for your CD28 related research.
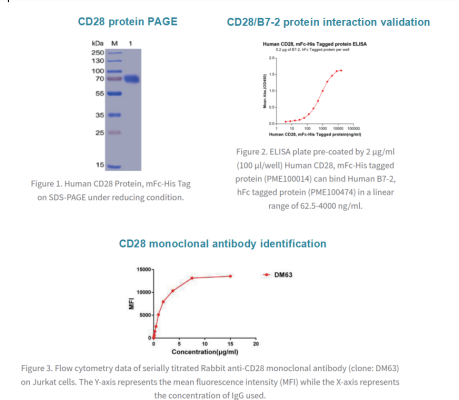
*Please click on the product names below to find detailed information.
Monoclonal antibodies
SKU: DME100063P Target: CD28
Application: Flow Cyt
Price: 100 test $550.00
Overexpression Stable Cell Line
SKU: CEL100041 Target: CD28
Application: FACS Data
Price:Inquiry
ECD Proteins
SKU: PME101387 Target: CD28
Price: 10μg $79.00; 50μg $281.00 ; 100μg $422.00
Monoclonal antibodies
SKU: DME100063B Target: CD28
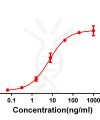
Application: ELISA; Flow Cyt
Price: 10μg $139.00 ; 100 μg $670.00 ; 500 μg $1999.00
Monoclonal antibodies
SKU: DME100063 Target: CD28
Application: ELISA; Flow Cyt
Price: 10μg $99.00 ; 100 μg $446.00 ; 500 μg $1340.00
ECD Proteins
SKU: PME-M100009 Target: CD28
Price: 10μg $72.00; 50μg $272.00; 100μg $409.00
ECD Proteins
SKU: PME100014 Target: CD28
Price: 10μg $96.00; 50μg $370.00 ; 100 μg $555.00