1. About Synthetic Nanodisc
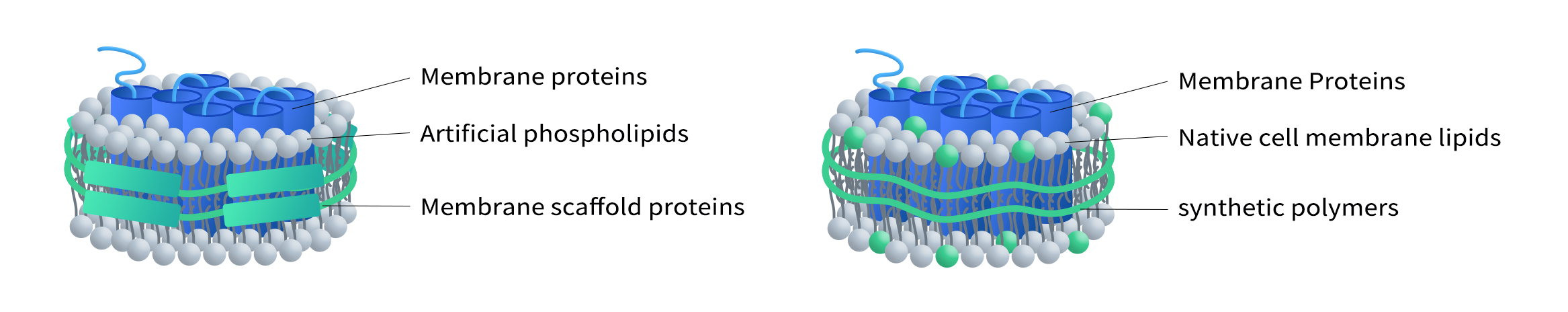
The lipid composition of traditional membrane scaffold protein (MSP) nanodiscs often differs from that of natural cell membranes, which can lead to deviations in membrane protein properties compared to their native environment. To address this limitation, DIMA has developed the Synthetic Nanodisc platform. This innovative technology enables the direct preparation of nanodiscs from intact cells. It utilizes stabilizers with both hydrophobic and hydrophilic properties. The hydrophobic surface of the stabilizer faces inward, interacting with the lipid bilayer, which allows membrane proteins to integrate into the nanodisc while preserving their native spatial conformation and activity. The hydrophilic exterior, on the other hand, ensures high solubility and stability of the nanodiscs in aqueous solutions.
In this process, synthetic polymers serve a dual purpose. They first dissolve cell membranes, functioning similarly to detergents, and then use natural cellular phospholipids to form the nanodisc structure around the membrane proteins. Additionally, DIMA employs mammalian expression systems to produce membrane proteins, ensuring that their structure, function, and post-translational modifications closely mirror their natural counterparts. This makes Synthetic Nanodisc an ideal tool for a wide range of applications in biochemistry and biomedical research.
To illustrate the effectiveness of this platform, we will explore how Synthetic Nanodisc technology enhances the expression and purification of the highly studied GPCR protein-GPR75.
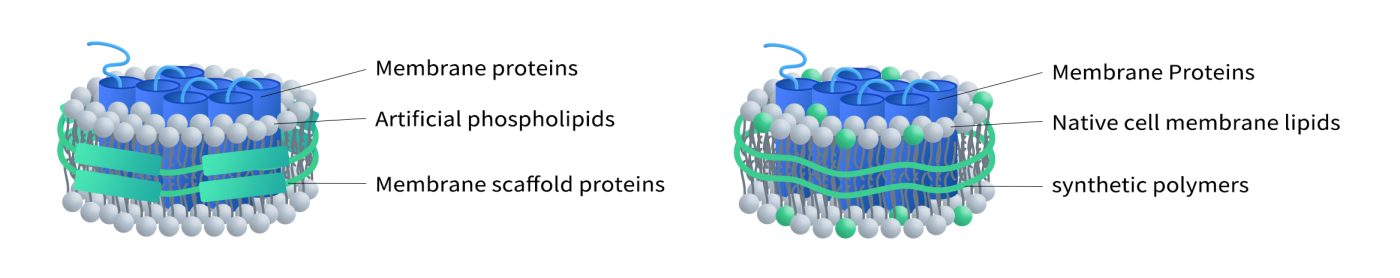
(Left: MSP Nanodisc; Right: Synthetic Nanodisc)
2. About GPR75
2.1 Structure of GPR75
GPR75, also known as G protein-coupled receptor 75, is a member of the GPCR family, specifically class A GPCR. GPCRs represent the largest family of cell surface receptors and are responsible for detecting a broad array of external signals, including hormones, neurotransmitters, lipids, and more. These receptors are characterized by seven transmembrane domains (TM1-7), connected by three extracellular loops (ECL1-3) and three intracellular loops (ICL1-3).
The third intracellular loop (ICL3) is particularly notable for its structural flexibility and variable length. ICL3 plays a critical role in interacting with G proteins, which are essential for intracellular signal transduction. Research has revealed that ICL3 is often disordered in crystallized GPCR structures. To stabilize GPCRs for crystallization, ICL3 is frequently replaced with soluble protein domains.
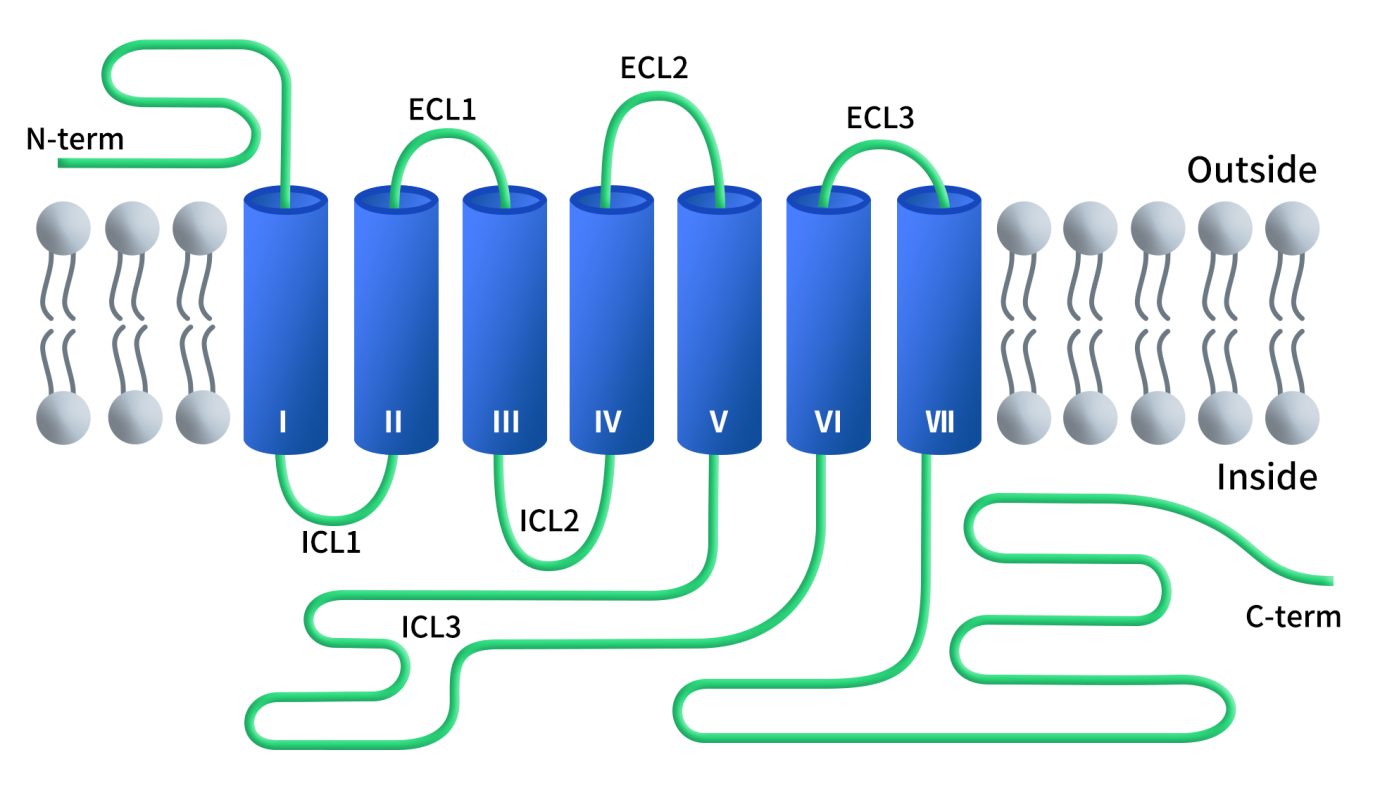
(Structural Simulation of GPR75 Protein)
2.2 GPR75 and Biomedical Development
Research indicates that GPR75 plays a critical role in various diseases, including obesity, cancer, and metabolic syndrome. To date, three ligands for GPR75 have been identified: 20-HETE, CCL5, and RANTES. Studies have shown that 20-HETE activates signaling pathways, such as PI3K/Akt and RAS/MAPK, through GPR75, resulting in a more invasive phenotype in prostate cancer cells. Moreover, the activation of the PI3K/Akt and RAS/MAPK pathways leads to the stimulation of NF-κB, which is involved in numerous pathways critical to cancer progression, including proliferation, migration, and apoptosis.
Further research suggests that inhibiting human GPR75 can enhance insulin sensitivity, improve glucose tolerance, and reduce fat storage in vivo. These findings position GPR75 as a potential therapeutic target for conditions such as obesity, metabolic syndrome, and cancer.
However, like many membrane proteins, GPCRs present significant challenges for researchers due to their intrinsic properties. The hydrophobic helical structures of GPCRs are embedded in the cell membrane, making them unstable in aqueous environments. Additionally, GPCR expression levels are generally low across most expression systems. Structural instability further complicates their study, as their conformations change in response to ligand binding, affecting binding affinity and complicating purification.
To address these challenges—such as low expression levels, conformational variability, and membrane protein-specific characteristics—DIMA has developed several solutions to enable the rapid and efficient purification of high-purity, stable membrane proteins in their native conformations. These solutions will be explored in the following sections.
3. GPR75-Nanodisc Solutions
3.1 Flag Tag
The Flag tag is a short and commonly used peptide sequence (usually DYKDDDDK) that allows efficient immunocapture using Anti-Flag antibodies. It offers several advantages in protein expression and purification:
Enhanced Purification Specificity: Anti-Flag antibodies exhibit high affinity and specificity for the tag, enabling selective capture of the target protein while minimizing nonspecific binding of impurities.
Minimal Interference with Protein Function: The Flag tag is relatively small and unlikely to disrupt the structure or function of membrane proteins, making it particularly useful for purifying transmembrane proteins, which are sensitive to structural and functional perturbations.
High Purification Efficiency: Flag tags enable efficient affinity purification using specific antibodies (e.g., Anti-Flag antibodies), simplifying the process. This provides a stable and efficient purification method, especially for transmembrane proteins that require a membrane environment for stability.
Compatibility with Various Expression Systems: The Flag tag is compatible with expression in multiple systems, including E. coli, yeast, insect cells, and mammalian cells. Regardless of the system, the Flag tag effectively binds to antibodies, facilitating purification.
High-Sensitivity Detection: Due to the high specificity of the Flag tag, Anti-Flag antibodies can be used to detect expressed membrane proteins. This allows even low-expressed membrane proteins to be effectively detected and enriched.
Given these advantages, the Flag tag has become a widely used tool for the purification of membrane proteins.
Human GPR75 full length protein-synthetic nanodisc (Cat# FLP100031)
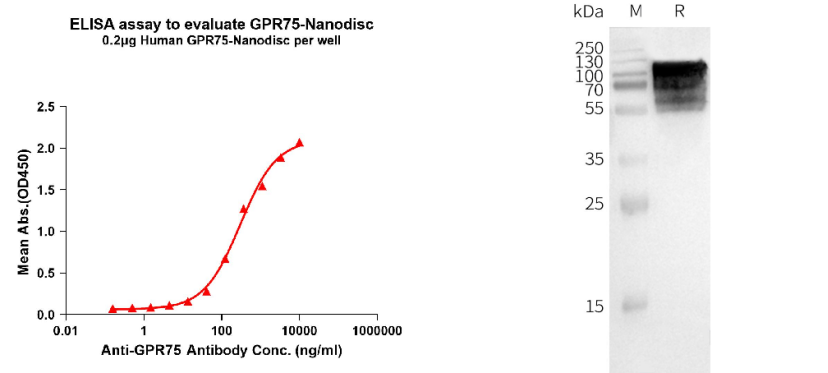
Left: ELISA analysis of Human GPR75-Nanodisc (Flag Tagged) with anti-GPR75 mAb (DMC100368); Right: WB analysis of Human GPR75-Nanodisc (Flag Tagged) with anti-Flag monoclonal antibody.
3.2 MBP Tag
The Maltose Binding Protein (MBP) tag is a widely used fusion tag in the expression and purification of membrane proteins. It provides several advantages in these processes:
Improved Solubility: Due to the hydrophobic and structurally complex nature of membrane proteins, they are prone to forming inclusion bodies. The MBP tag offers a less hydrophobic external structure, promoting stability in solution and reducing the formation of inclusion bodies.
Enhanced Expression Levels: MBP tags improve the folding and solubility of target membrane proteins, significantly increasing their expression levels. Many membrane proteins show low expression when untagged, but fusing them with MBP can achieve high-level expression in systems like E. coli.
Simplified Purification Process: MBP tags enable affinity chromatography purification. During purification, MBP binds specifically to maltose, allowing the fusion protein to be easily purified using maltose-containing resins. This process is straightforward, achieves high purity, and effectively removes most contaminant proteins through MBP-specific binding.
Removability: MBP tags can be cleaved using specific proteases, such as trypsin or His-tagged proteases, allowing for the recovery of pure target proteins for downstream applications. If the MBP tag interferes with the function or structure of the membrane protein, its removal restores the biological activity of the target protein.
Stability and Folding Support: In some cases, MBP tags assist membrane proteins in proper folding, maintaining their functional and structural stability. This prevents protein degradation or inactivation during purification, which is crucial for structural analyses. (e.g., X-ray crystallography or cryo-EM and functional studies)
Human MBP-GPR75 full length protein-synthetic nanodisc (Cat# FLP110031)
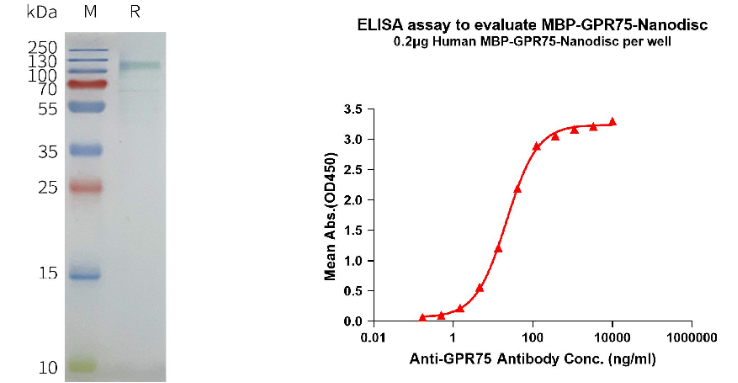
Left: Human MBP-GPR75-Nanodisc with N-MBP Tag, C-Flag Tag on SDS-PAGE; Right: ELISA analysis of N-MBP Tag, C-Flag Tag MBP-GPR75-Nanodisc with anti-GPR75 monoclonal antibody (DMC100368).
3.3 Strep Tag
The Strep tag (also known as the Streptavidin tag) is a widely used affinity tag that exploits the high-affinity interaction between streptavidin and its ligand, biotin. It is commonly applied in protein purification and capture. The advantages of using the Strep tag in membrane protein expression and purification include:
High-Affinity Purification: The Strep tag has an extremely high affinity (Kd ≈ 10⁻¹⁴ M) for streptavidin resins, enabling effective purification of target proteins, even at low concentrations. This allows for the recovery of high-purity target proteins without disrupting their native conformation.
Small Size: The Strep tag is relatively small (approximately 8 kDa), causing minimal interference with the structure and function of membrane proteins. It can often remain attached to the protein during downstream experiments without the need for removal.
Ease of Detection: The Strep tag can bind to labeled streptavidin (e.g., fluorescent or enzyme-labeled), making it easy to detect and analyze membrane protein expression and purity. This is especially useful for monitoring membrane protein expression and for use in downstream applications.
Compatibility with Multiple Detection Systems: Strep-tagged proteins are highly compatible with a variety of detection systems, including ELISA, surface plasmon resonance (SPR), and biolayer interferometry (BLI), making them ideal for protein immobilization and detection.
Human GPR75-Strep full length protein-synthetic nanodisc (Cat# FLP120031)
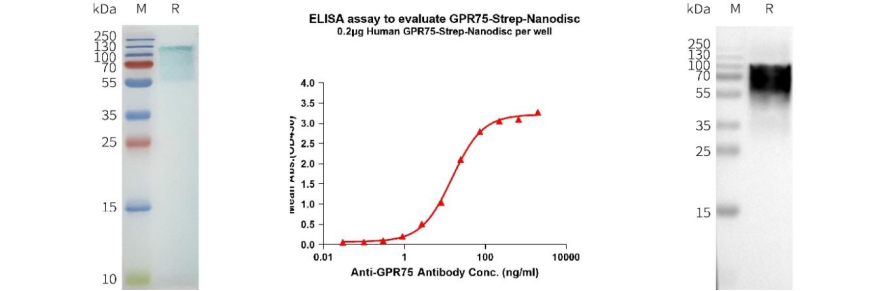
Left: Human GPR75-Strep-Nanodisc, C-Flag and Strep Tag on SDS-PAGE; Middle: ELISA analysis of Human GPR75-Strep-Nanodisc, C-Flag and Strep Tag using anti-GPR75 monoclonal antibody (DMC100368); Right: WB analysis of GPR75-Strep-Nanodisc with anti-GPR75 monoclonal antibody (DMC100368).
3.4 β2AR and BRIL Fusion Protein
The β2-adrenergic receptor (β2AR) is a member of the β-adrenergic receptor family and belongs to the class of G protein-coupled receptors (GPCRs). A major challenge in purifying GPCRs is maintaining their stability and functionality, as they depend on the lipid environment within the membrane. Studies have shown that modifying the N-terminal residues of GPCRs can alter the receptor’s embedding orientation in the membrane, which in turn affects its ligand-binding affinity. Both GPR75 and β2AR share structural similarities as members of the GPCR family, and replacing the N-terminus of GPR75 with that of β2AR has been shown to enhance its expression levels.
BRIL, derived from bacterial cytochrome b562, features a tightly folded α-helical structure. Mutations in BRIL further stabilize its structure, making it highly resistant to variations in temperature and solution conditions. BRIL exhibits a dual characteristic: while it is flexible in the heme-binding region, its distal region is highly stable and rigid. This structural duality facilitates rapid folding kinetics and overall stability.
These properties make BRIL an ideal fusion partner for membrane proteins, particularly in the loop regions between transmembrane helices. BRIL can accommodate minor lateral displacements between the helices of the two proteins, contributing to both folding kinetics and stability. Therefore, replacing the original intracellular loop 3 (ICL3, residues 237-306) of GPR75 with the BRIL sequence enhances the receptor’s overall stability
Human β2AR-GPR75-BRIL full length protein-synthetic nanodisc (Cat#FLP110031A)
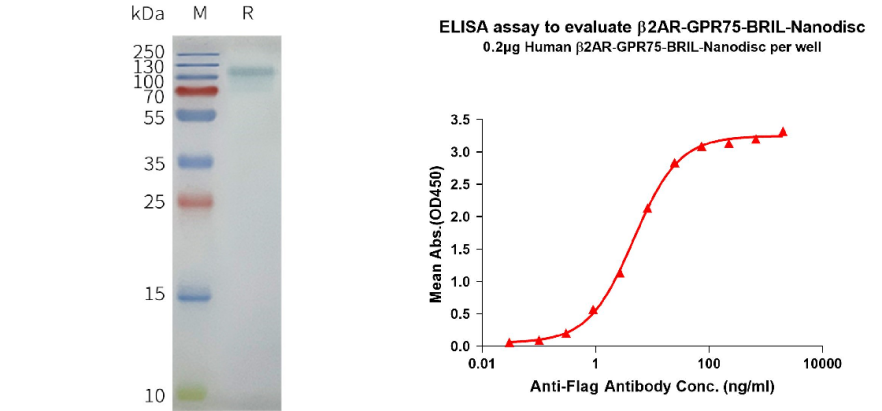
Left: Human β2AR-GPR75-BRIL-Nanodisc, Flag Tag on SDS-PAGE; Right: ELISA analysis of Flag Tag β2AR-GPR75-BRIL-Nanodisc with anti-Flag monoclonal antibody.
4. Other GPR75 Products
To support biopharmaceutical development, DIMA has developed a comprehensive range of products targeting GPR75. These include extracellular domain recombinant proteins, full-length membrane proteins, proprietary monoclonal antibodies, Biotinylated and PE-conjugated antibodies, and stable cell lines.
In addition to the existing products, DIMA offers professional customization services for proteins (different species/tags) and antibodies (humanization/affinity maturation) to meet diverse customer requirements. We also provide a single B-cell seed library for the GPR75 target, enabling rapid screening of lead antibody candidates within approximately 28 days. For more information, please feel free to contact us.