On November 20, 2024, Jazz Pharmaceuticals announced that the bispecific antibody Zanidatamab had received accelerated approval from the FDA for the treatment of adult patients with previously treated, unresectable, or metastatic HER2-positive (HER2+, defined as IHC 3+) biliary tract cancer (BTC). Zanidatamab is the first approved HER2-targeting bispecific antibody with dual epitope specificity.
On December 4, 2024, Merus NV announced that the FDA approved BIZENGRI® (zenocutuzumab-zbco) for the treatment of adult patients with advanced, unresectable, or metastatic pancreatic cancer (PDAC) or non-small cell lung cancer (NSCLC) carrying NRG1 gene fusions, whose disease progressed during or after prior systemic therapy. Zenocutuzumab is not only the world’s first approved HER2×HER3 bispecific antibody but also the first FDA-approved therapy specifically targeting NRG1+ advanced unresectable or metastatic PDAC and NSCLC.
HER2 (human epidermal growth factor receptor 2) is a common molecular target in various cancers, especially in breast cancer and gastric cancer. Next, let’s take a closer look at HER2 and its related clinical drugs.
1. About HER2
1.1 Structure and Distribution of HER2
The human epidermal growth factor receptor (HER) family consists of four transmembrane tyrosine kinase receptors: EGFR (also known as HER1), HER2, HER3, and HER4. These receptors can dimerize with each other to mediate cell growth, differentiation, and survival. The four HER receptors form ten different homodimers and heterodimers, integrating complex biological signaling events. Overexpression of HER2 has been shown to be associated with aggressive tumors, making it a crucial target for anticancer drug development.
Ligand-induced homo- or heterodimerization of HER proteins triggers downstream phosphorylation signaling cascades, stimulating cell growth, proliferation, and differentiation. Among these, EGFR and HER2 are considered the most potent oncogenic proteins. Their overexpression is linked to various cancers, including breast cancer, lung cancer, and gastroesophageal cancer.
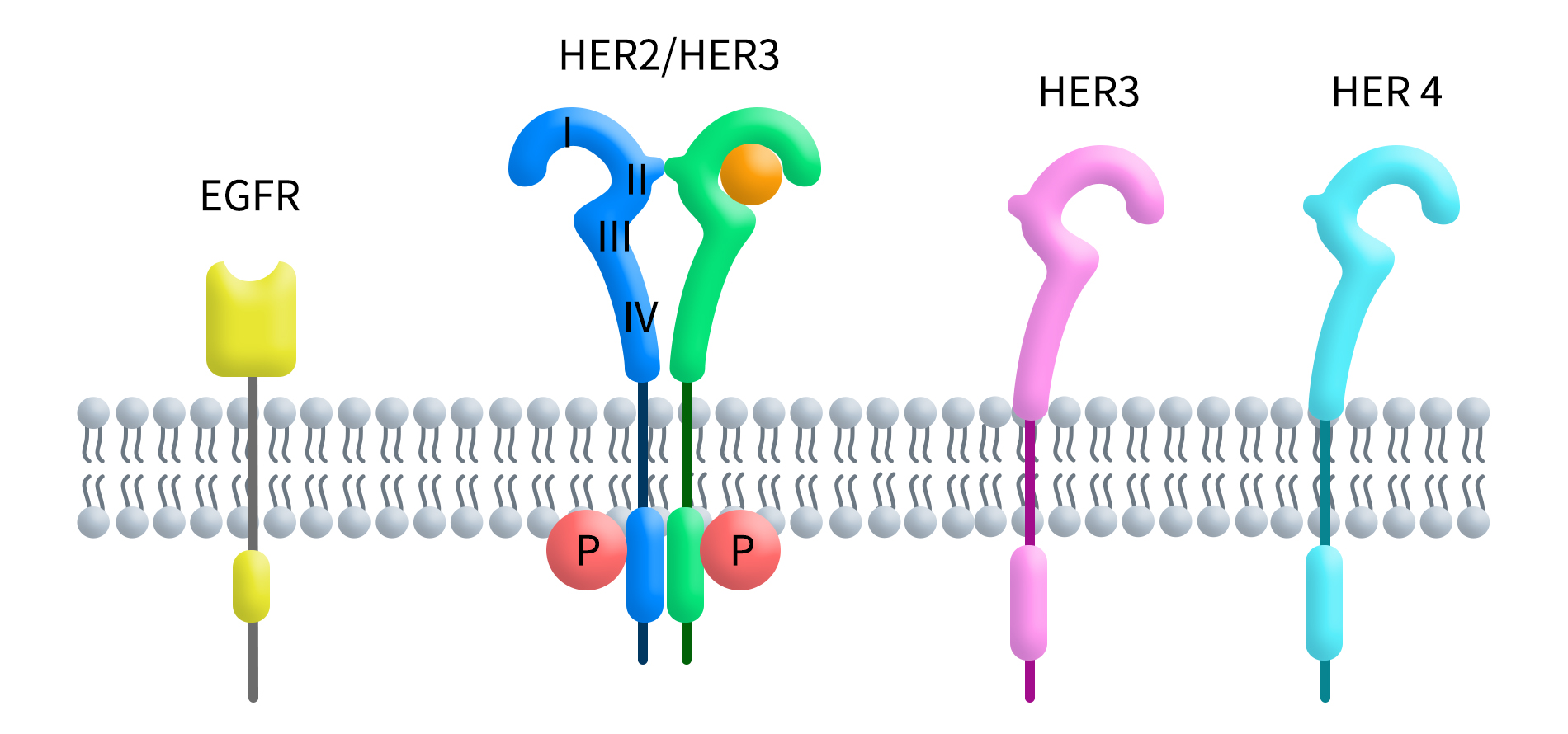
(Cite form HER2-Positive Gastric Cancer: The Role of Immunotherapy and Novel Therapeutic Strategies)
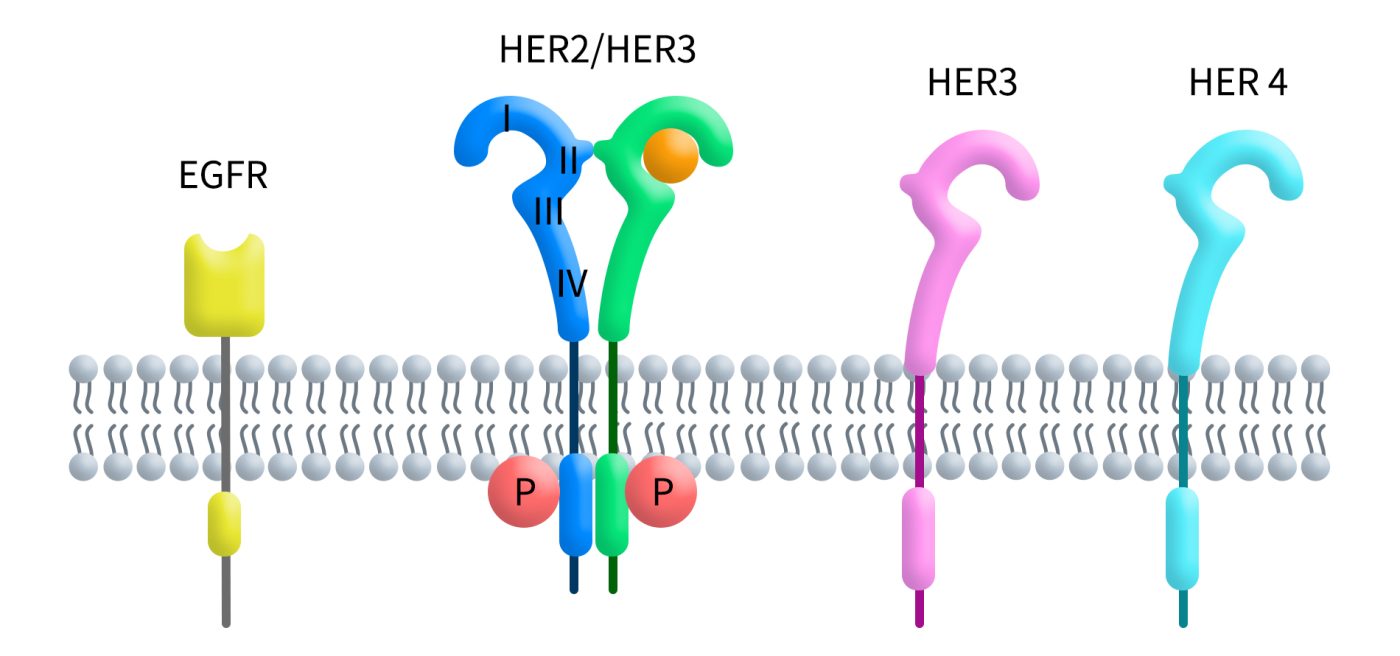
Research has shown that the extracellular domain (ECD) of the HER receptor family consists of four subdomains (I-IV) and can exist in only two conformations: the tethered form and the extended form. In the tethered form, interactions between subdomain II and subdomain IV prevent the ECD from mediating dimerization. However, in the extended form, the receptor’s dimerization elements are fully exposed, allowing dimerization and signal transduction.
What makes HER2 unique is that it constitutively exists in the extended form due to direct interactions between subdomains I and III, which stabilize its structure. This explains why HER2 is the preferred dimerization partner for other HER family members and highlights its critical role in tumor progression.
1.2 HER2-Mediated Signaling Pathways
HER2’s extracellular domain lacks a known ligand and is activated through the formation of homodimers or heterodimers. These dimers lead to phosphorylation of tyrosine kinase residues in the cytoplasmic domain, which serve as docking sites for proteins that activate downstream PI3K and MAPK signaling pathways. This activation drives cell cycle progression and proliferation.
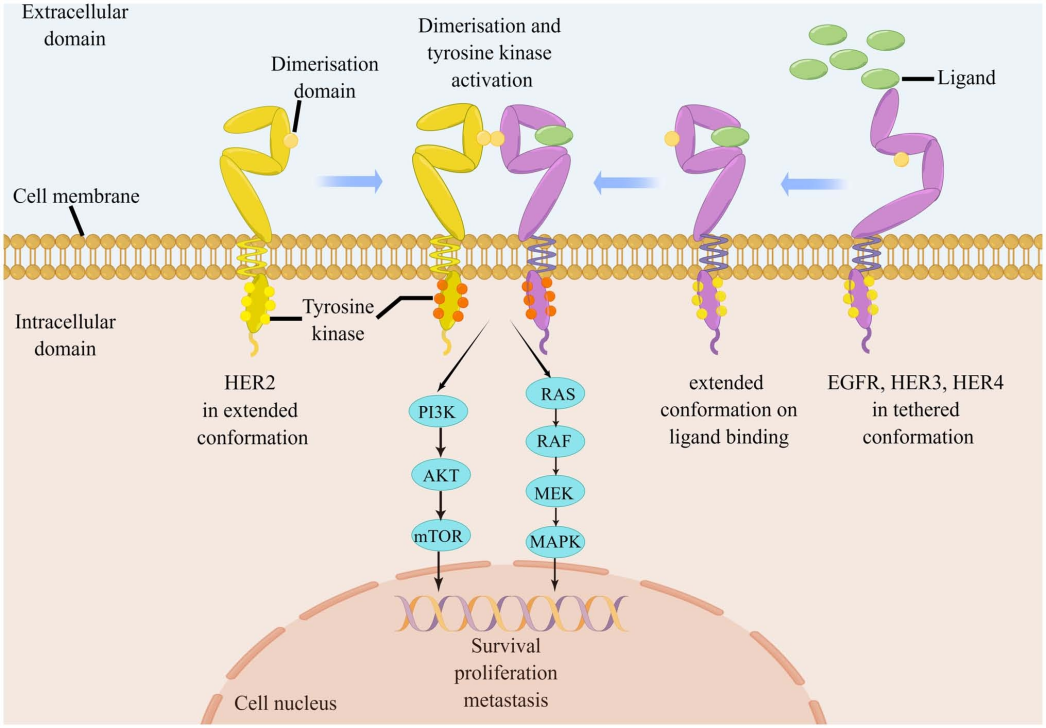
(Cite form HER2(+) advanced gastric cancer: Current state and opportunities (Review))
2. HER2 Clinical Drugs
Currently, HER2-targeted therapies primarily include monoclonal antibodies, small-molecule tyrosine kinase inhibitors (TKIs), and antibody-drug conjugates (ADCs). The mechanisms of action of antibodies mainly involve two aspects:
- Binding to the extracellular domain of the HER2 protein to prevent the formation of HER2-containing heterodimers, thereby modulating downstream effectors of the ERBB2 signaling pathway.
- Recruiting extracellular immune cells to induce antibody-dependent cell-mediated cytotoxicity (ADCC).
Representative drugs include trastuzumab, pertuzumab, and ZW25. ZW25 is a bispecific antibody that targets both subdomain II (the pertuzumab binding site) and subdomain IV (the trastuzumab binding site) of HER2’s extracellular region, enhancing ADCC activation.
(Cite form HER2-targeted therapies in cancer: a systematic review.)
2.1 Monoclonal Antibody Therapies
Trastuzumab (Herceptin):
Trastuzumab is one of the earliest HER2-targeted therapies. As a monoclonal antibody, it binds to the HER2 receptor, inhibiting its downstream signaling pathways and preventing cancer cell proliferation. Trastuzumab has been widely used in the treatment of HER2-positive breast cancer and gastric cancer.
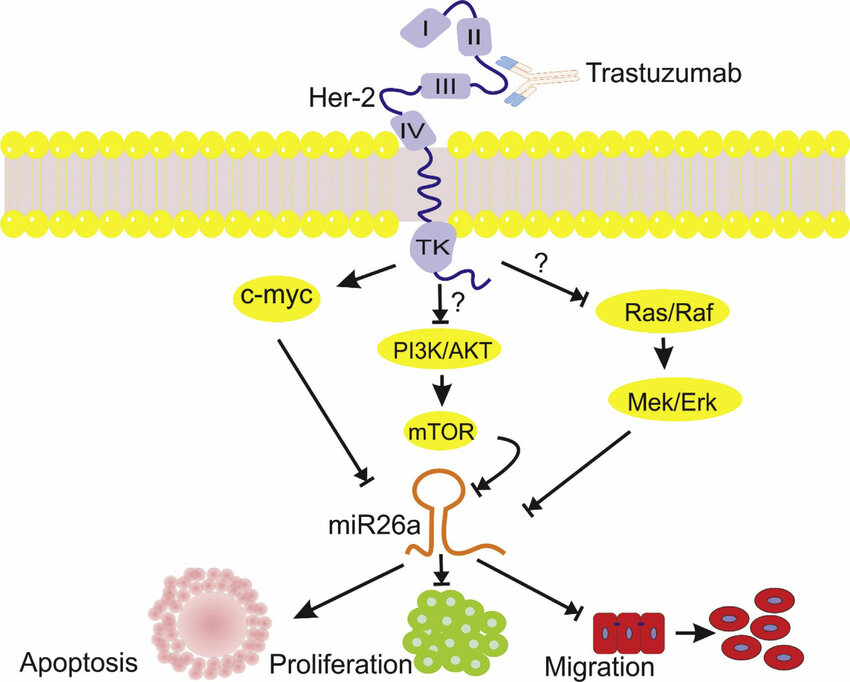
(Cite form MicroRNA-26a-5p as a potential predictive factor for determining the effectiveness of trastuzumab therapy in HER-2 positive breast cancer patients.)
Perjeta (Pertuzumab):
Similar to trastuzumab, pertuzumab is also a monoclonal antibody targeting HER2. However, it works through a different mechanism—it blocks HER2 dimerization while also enhancing the immune system’s ability to attack cancer cells.
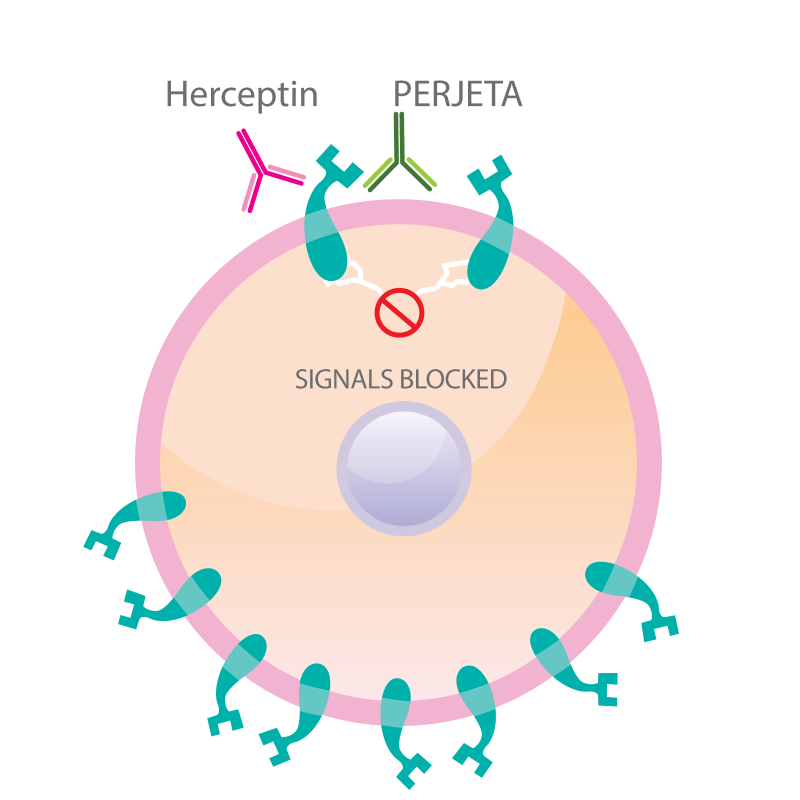
(Cite from Slideshare net)
2.2 Small Molecule Inhibitors
Lapatinib: Lapatinib is a small-molecule tyrosine kinase inhibitor that targets the tyrosine kinase activity of the HER2 receptor. It is administered orally and is used for the treatment of HER2-positive breast cancer. Lapatinib can be used alone or in combination with other therapies to enhance its efficacy.
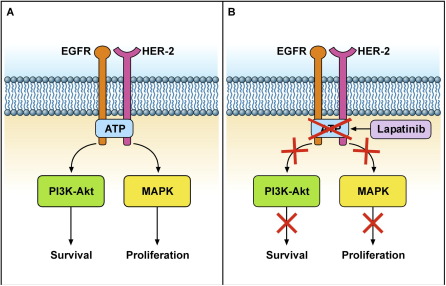
(Cite from Mechanisms of lapatinib resistance in HER2-driven breast cancer.)
Neratinib: Neratinib is another HER2-targeted tyrosine kinase inhibitor, commonly used for the treatment of HER2-positive breast cancer, especially in patients who experience disease progression after trastuzumab therapy.
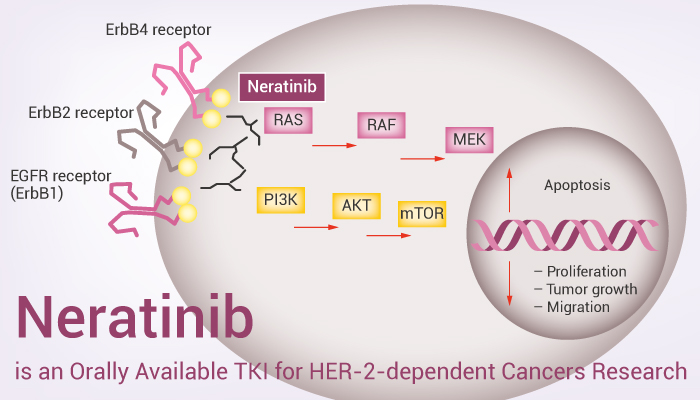
(Cite from Network of Cancer Research.)
2.3 Antibody-Drug Conjugates (ADC)
Enhertu (DS-8201):
Enhertu is a highly promising HER2-targeted ADC that has demonstrated significant efficacy in multiple clinical trials. Compared to traditional HER2-targeted therapies, Enhertu exhibits stronger anticancer activity, especially in patients who have developed resistance to trastuzumab. This drug combines a HER2-targeting antibody with a potent cytotoxic agent, allowing for the direct delivery of the toxin to tumor cells.
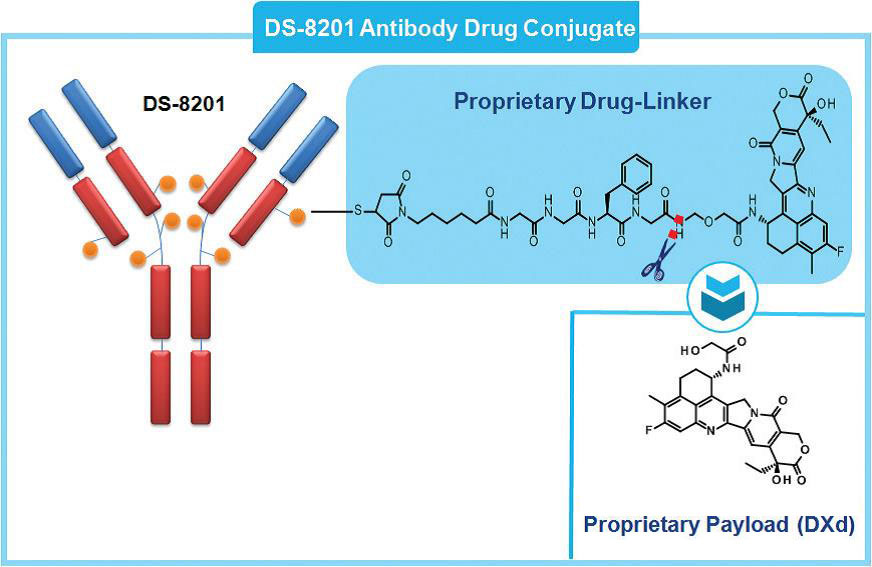
(Cite from Ceative-biolabs)
Kadcyla (T-DM1):
Kadcyla is a HER2-targeted antibody-drug conjugate (ADC) that combines trastuzumab with the cytotoxic agent emtansine (DM1). This drug delivers the cytotoxin directly to HER2-positive tumor cells, enhancing efficacy while reducing damage to normal cells.
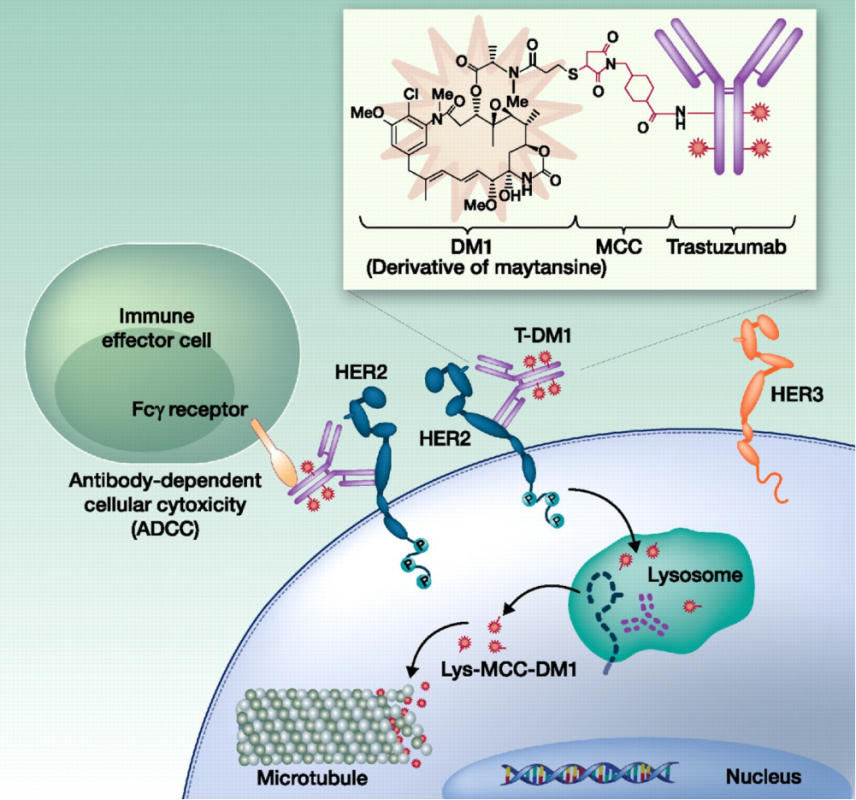
(Cite from Profiling and targeting HER2-positive breast cancer using trastuzumab emtansine)
The development of HER2-targeted therapies has made significant progress. However, resistance remains one of the major challenges in treatment, as adaptive changes in HER2-positive cancers during therapy can lead to the failure of anti-HER2 drugs. Therefore, developing new therapies to overcome drug resistance remains a key research focus.
3. DIMA HER2-Related Products Supporting Drug Development
DIMA Biotechnology Co., Ltd. is a biotechnology company specializing in preclinical research products and services for druggable targets. We currently offer a range of ready-to-use HER2-targeted products, including recombinant proteins, monoclonal antibodies, reference antibodies, biotin/PE-labeled antibodies, and CDX tissue sections.
Additionally, we provide comprehensive services, such as protein/antibody customization, antibody humanization, antibody affinity maturation, and functional cell line development. Furthermore, we have established a B-cell seed library for the HER2 target, allowing us to screen lead antibody candidates within just 20 days.
For more information, please feel free to contact us.
- Proteins & Antibodies
- DIMA HER2 Lead Molecule Research Progress

References
1. Hao, Y., et al., Cryo-EM Structure of HER2-trastuzumab-pertuzumab complex. PLoS One, 2019. 14(5): p. e0216095.
2. Bai, X., et al., Structure and dynamics of the EGFR/HER2 heterodimer. Cell Discov, 2023. 9(1): p. 18.
3. Pous, A., et al., HER2-Positive Gastric Cancer: The Role of Immunotherapy and Novel Therapeutic Strategies. Int J Mol Sci, 2023. 24(14).
4. Hu, H.H., et al., HER2(+) advanced gastric cancer: Current state and opportunities (Review). Int J Oncol, 2024. 64(4).
5. Zhu, K., et al., HER2-targeted therapies in cancer: a systematic review. Biomark Res, 2024. 12(1): p. 16.